Electrolysis of Water :)
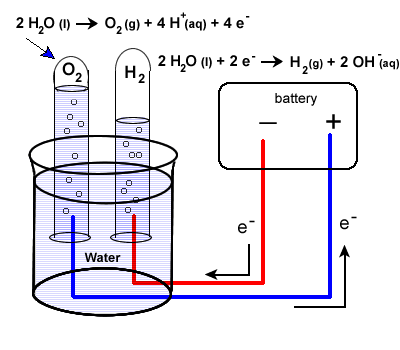 13.44 liters of oxygen gas was produced at STP after electrolysis of water. What are the mass of the decomposed water and the volume of the hydrogen produced at STP?
13.44 liters of oxygen gas was produced at STP after electrolysis of water. What are the mass of the decomposed water and the volume of the hydrogen produced at STP?
Note: The atomic weights of hydrogen and oxygen are 1 and 16, respectively.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The balanced reaction of decomposition of water is given by:
2 H X 2 O ( l ) 2 H X 2 ( g ) + O X 2 ( g )
At same temperature and pressure, volume of a gas is proportional to its moles. Therefore, the volume of H X 2 produced in this case is twice that of O X 2 or 1 3 . 4 4 × 2 = 26.88 L .
At STP, 1 mol of gas has a volume of 22,4 L , therefore, 13.44 L of O X 2 is 2 2 . 4 1 3 . 4 4 = 0.6 mol . Since 2 mol of H X 2 O produces 1 mol of O X 2 . Therefore, the mole of H X 2 O decomposed was 0 . 6 × 2 = 1.2 mol = 1 . 2 × ( 1 × 2 + 1 6 ) = 1 . 2 × 1 8 = 21.6 g .
The answer is 21.6 g, 26.88 L