Endothermic To Exothermic
 Source : Resonance AIOT
Source : Resonance AIOT
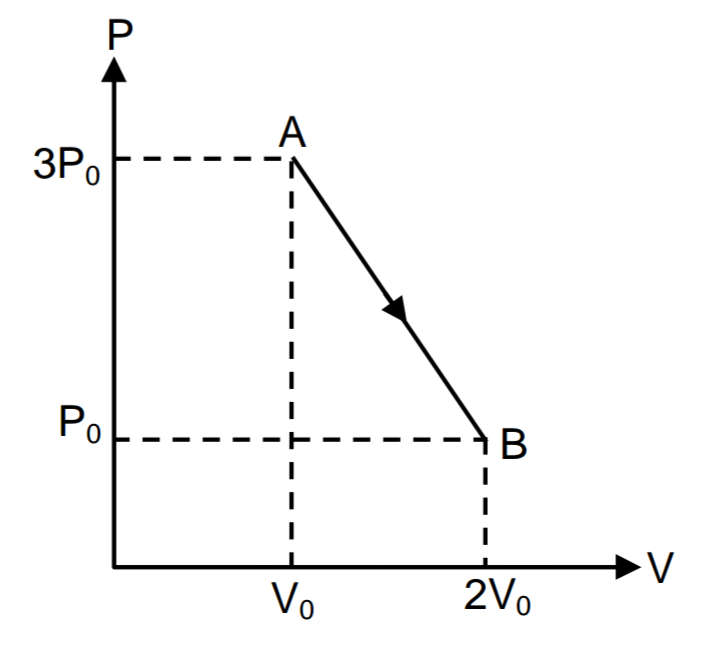
An ideal diatomic gas undergoes process A B for which P V indicator diagram is shown. At some time during this process, it changes from being endothermic to being exothermic. At that time, the volume of the gas is 7 2 5 λ V 0 . Find λ .
The answer is 21.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
1 solution
We look for a point on A B that is tangent to the adiabatic curve P V γ = k , or P = k V − γ , with γ = 7 / 5 . The derivative is d P / d V = − γ k V − γ − 1 = − γ P / V . This derivative must match the slope of A B , which is − 2 P 0 / V 0 .
Thus d V d P = − 5 7 V P = − 2 V 0 P 0 , so that 7 P 0 P − 1 0 V 0 V = 0 ; but we also have for line A B P 0 P + 2 V 0 V = 5 . Multiply the last equation by 7 and subtract the first to find 2 4 V 0 V = 3 5 , so that V = 2 4 3 5 V 0 = 2 4 ⋅ 3 5 ⋅ 7 ⋅ 3 ⋅ V 0 . Thus the answer is 2 1 .
Log in to reply
Thanks , I commited a silly mistake while solving :)
Equation of the straight line AB is :
P = 5 P 0 − V 0 2 P 0 V
Q (Heat) as a function of volume is :
Q = 2 3 5 P 0 V − V 0 6 P 0 V 2 − 2 2 3 P 0 V 0
Above equation is a downward parabola with apex occuring at V ′ = 2 4 3 5 V 0 . After V ′ , Q is decreasing (Heat must have started evolving after the apex leading to a fall in curve)
Hence V ′ must be the required volume .
V ′ = 2 4 3 5 V 0