Failing Hückel
 Cyclodecapentaene or [10]annulene is a compound which is unstable, despite its resemblance to benzene, an aromatic compound. What criterion of Hückel's rule does cyclodecapentaene fail to meet?
Cyclodecapentaene or [10]annulene is a compound which is unstable, despite its resemblance to benzene, an aromatic compound. What criterion of Hückel's rule does cyclodecapentaene fail to meet?
-
It is not cyclic.
-
It is not planar.
-
It does not have electrons in its delocalised, conjugated -orbital cloud.
-
Not all its atoms participate in delocalising electrons.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
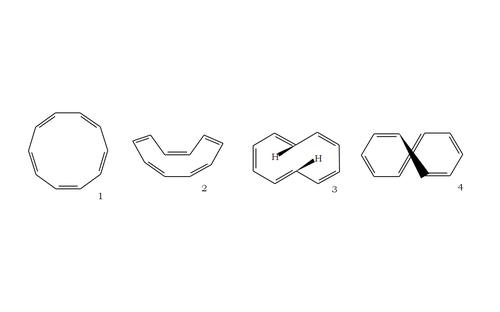
Let's observe the possible structures of cyclodecapentaene.
The first structure has bond angles of 1 4 4 ∘ , making it unfavourable as compared to the ideal bond angle of 1 2 0 ∘ predicted by VSEPR.
The second structure relieves the bond strain from the first structure but is still unstable due to the all-cis conformation.
The third structure would be stable if not for the steric repulsion from the internal hydrogen atoms.
The fourth structure, then is the most stable; however, it is clearly not planar and as such is not aromatic.