Free electron in magnetic field (with a spark of quantum theory)
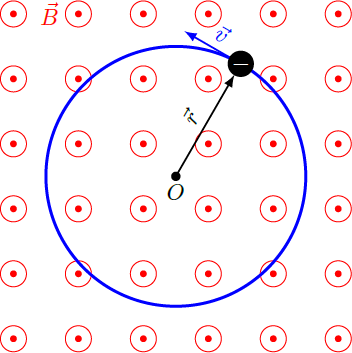
A free electron with mass and charge , which moves in the xy-plane, is forced by a homogeneous magnetic field in the z-direction on a circular path with radius . This phenomenon can be demonstrated in a classroom experiment using an electron tube (Teltron tube), in which the electrons stimulate gas molecules through collisions and make them glow. At a magnetic field of 1.3 millitesla, the circular tracer of the electrons in this experiment has a typical radius of a few centimeters. How big is the smallest possible orbital radius for electrons in the same magnetic field according to the quantum theory?
Hints: According to the Bohr-Sommerfeld quantization condition, only those circular orbits are permitted for the electron for which the orbital angular momentum is just a half-integer multiple of the reduced Planck's constant : The relevant physical constants are
This is just a Semiclassical approach that does not fully describe the physical behavior of the electron. However, the quantization condition gives us classical orbits that correspond to different quantum mechanical states and describe the mean value of the radial distance .
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
According to classical Newtons laws, the electron orbit results from the balance of lorentz force F L and centrifugal force F cf : F L = ( − e ) ⋅ v × B ⇒ e v B ⇒ v = − F cf = − m r v 2 e r = m r v 2 = m e B r If we use this result and insert it into the quantization condition, we obtain discrete values r n for the orbit radius: L = m v r = e B r 2 ⇒ r n = ℏ ( n + 2 1 ) = e B ℏ ( n + 2 1 ) The smallest radius for the case n = 0 yields r 0 = 2 e B ℏ = 5 . 0 3 ⋅ 1 0 − 7 m ≈ 0 . 5 μ m