A chemistry problem by Aakhyat Singh
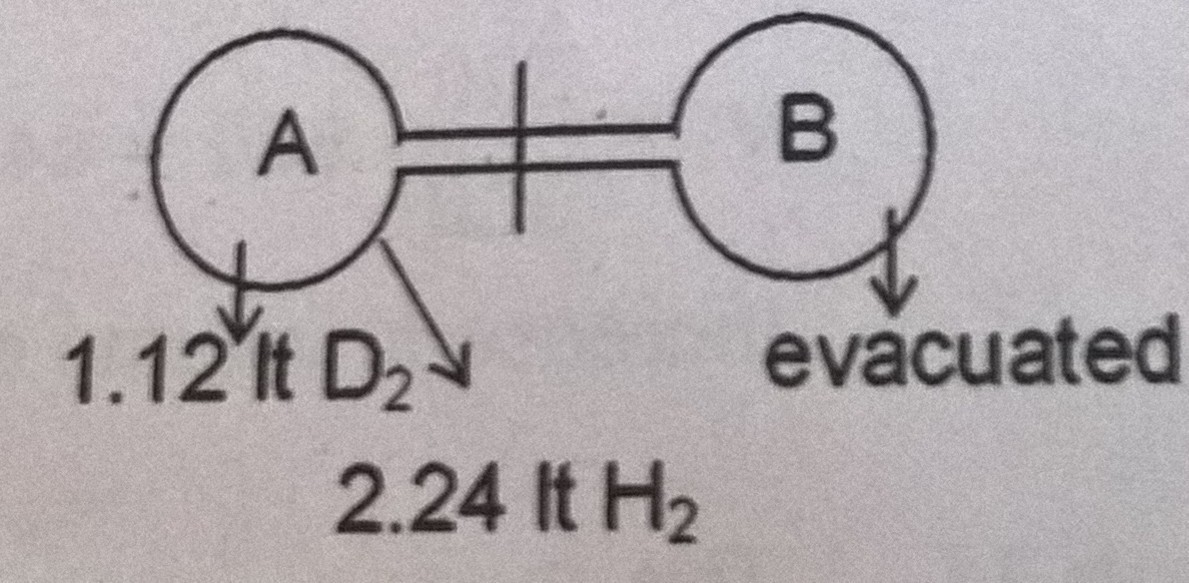 The above shown experiment is done at NTP. The stop cock is opened for a certain time and then closed. After effusion the bulb A contains 0.1 g D2. Find out the number of moles of H2 in bulb B.
The above shown experiment is done at NTP. The stop cock is opened for a certain time and then closed. After effusion the bulb A contains 0.1 g D2. Find out the number of moles of H2 in bulb B.
The answer is 2.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!