How can I know this period?
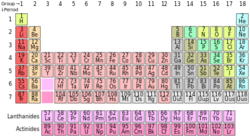 The above is the periodic table of the elements which is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers, electron configurations and recurring chemical properties. Which of the following determines the groups (1 to 2) and (13 to 18)?
The above is the periodic table of the elements which is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers, electron configurations and recurring chemical properties. Which of the following determines the groups (1 to 2) and (13 to 18)?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Each corresponding electron shell holds 2n^(2) electrons eg(2,8,18,32,50) In Group 1, each has 1 electron in the outer shell (Hence Valence Charge of 1) eg. Hydrogen is 1,0,0,0,0 Lithium is 2,1,0,0,0 Sodium is 2,8,1 ,0,0 This is also true for Group 2 which has 2 electrons in each of the outer shells Meanwhile Group 18(8 for simplicity) has a full outer shell of electrons (8 electrons) Each group before that has 1 less electron in it's outer shell
The amount of electrons in each element is dependent on it's atomic number(protons) hence groups 1,2,13-18 each have 1-8 electrons in their outer shell. Thus, the valence electron number determines the groups of the periodic table.