This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
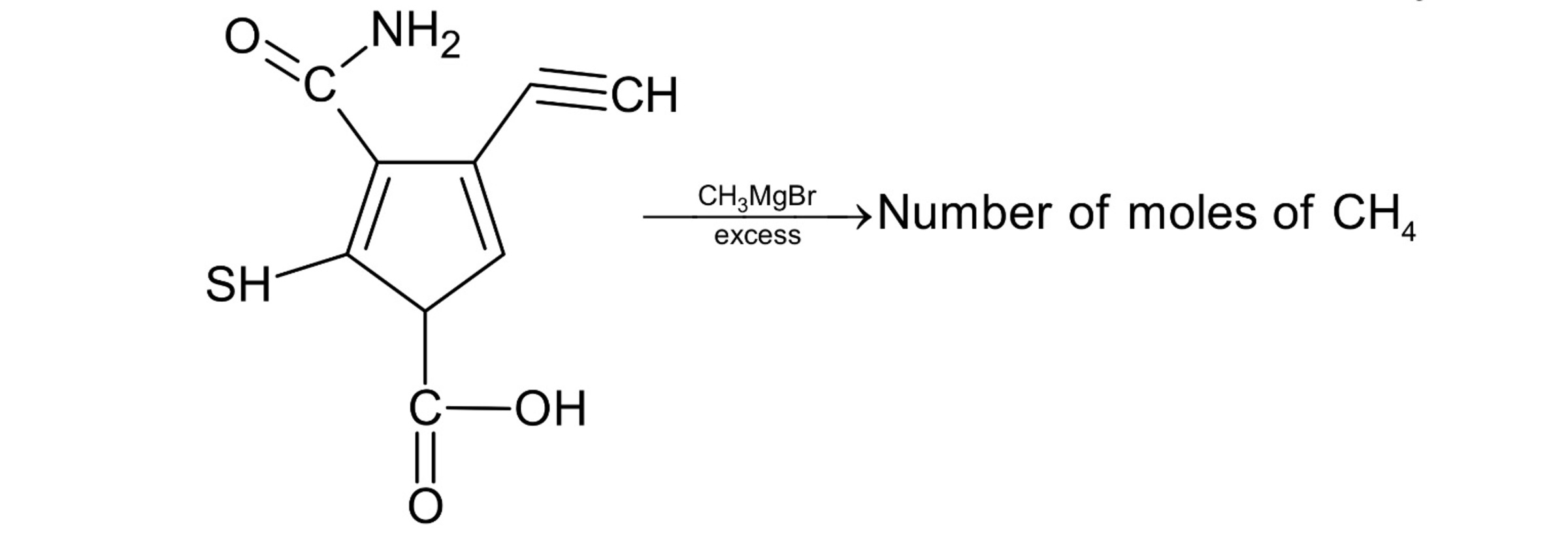
Grignard Reagent (RMgX) Where R is an alkyl or phenyl group and X is an halogen(normally chlorine and bromine) is a widely used reagent in organic chemistry. As magnesium is an electropositive element carrying a positve 2 charge hence R Group must also carry a unit negative charge that means that in RMgX Carbon has carbanion character (which is a very strong base) . Basicity decreases along a period as electronegativity increases so tendency to loose electrons decreases. Hence it can abstract slightly acidic hydrogen also (like from terminal alkynes) . Hence it can take H+ from Nitrogen,alkyne,Thioalcohol and carboxylic acid and will form methane. (A similar reaction is corey house synthesis also albeit it uses organo lithium compounds)