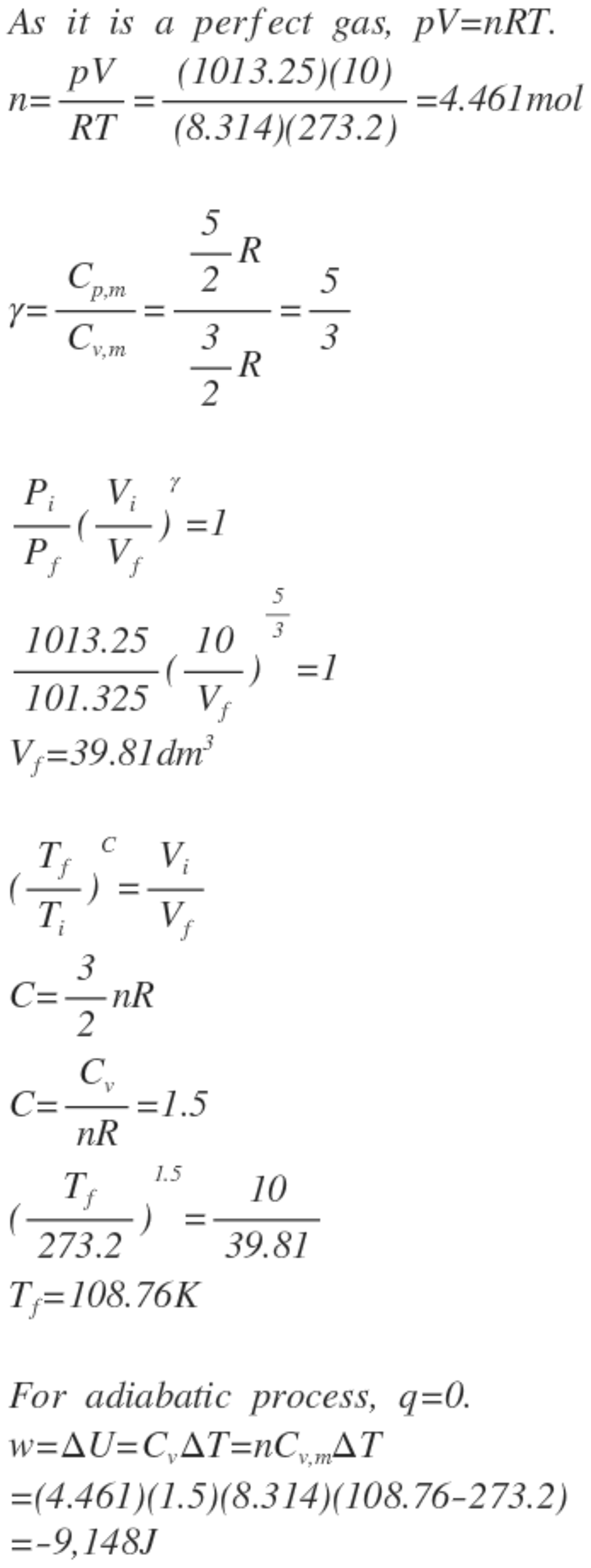How much effort should be put in?
You were given of gas, which you would use to go through an adiabatic reversible expansion. The gas was at and initially, at the end it was at . Assume the gas behaved as a perfect gas. How much work had to be put in?
Present your answer in Joule, and round it to the nearest integer.
The answer is -9148.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.