Ideal gas expansion
Chemistry
Level
2
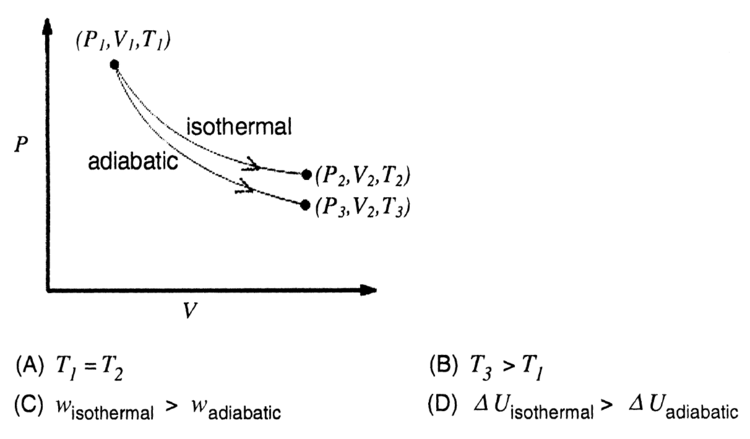 The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is (are) correct?
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is (are) correct?
B and D
A and B
A, C, and D
B and C
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
For isothermal process t remains constant And U1 = 0 in isothermal whereas it is equal to work in adiabatic i.e., U2 = w and w = -P(V2 - V1) and in expansion V2 - V1is positive therefore w and U2 becomes negative which make U1>U2 . Well I really think that the third one is incorrect