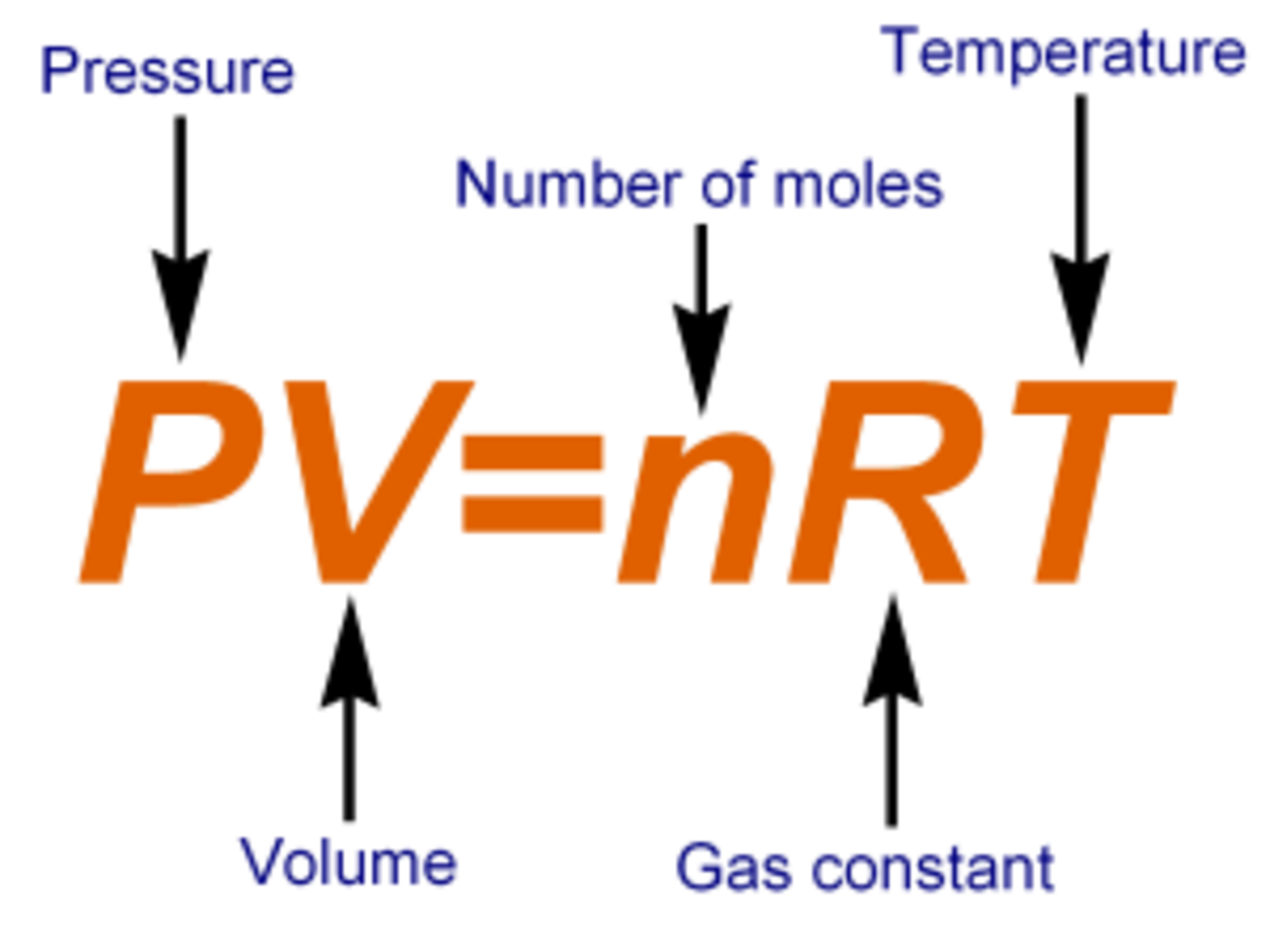
Ideal Gases
2 moles of an ideal gas "A" is mixed with 5 moles of another non reacting gas "B" in a container of volume "V" . If the temperature of the gas is kept constant and the volume is doubled , then which of the following statements is false?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!