Imidazole
Chemistry
Level
1
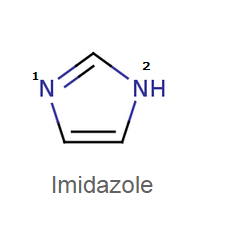 Which nitrogen is more acidic?
Which nitrogen is more acidic?
1
2
both are equally acidic
nothing can be said
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
First one is most basic because it's lone pairs are not in conjugation because they are not in the plane of the π orbitals of the s p 2 hybrid carbons of the ring. And so they are free to be donated!