Inspired By A JEE 2014 Question
The radiation corresponding to the transition of hydrogen atom falls on a metal surface to produce photoelectrons. These electrons are made to enter an electric field of as shown in the figure. If the electrons just change their direction of motion after 1 second, then the work function of the metal is close to which of the following choices?
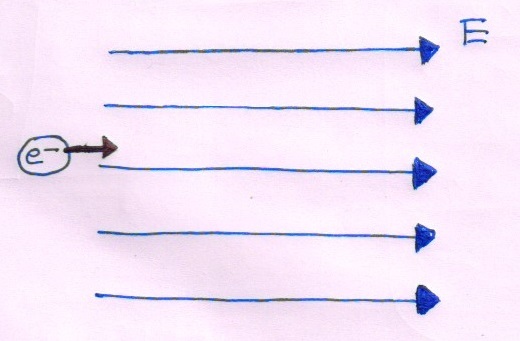
Details and Assumptions :
-
Take the mass of electron, ,
-
The charge on electron, and .
-
The Ionization energy of Hydrogen atom in ground state is
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The acceleration of the electron is a = − m e e E . If it reaches zero speed after time t , it's initial velocity must have been v 0 = − a t = m e e E t , which means it had a kinetic energy of K 0 = 2 1 m e v 2 = 2 m e e 2 E 2 t 2 = 8 . 8 e V . (To find the energy in eV, I simply left one factor e out.)
The photon that was released had energy E γ = − ( E 1 − E 2 ) = 1 2 Ry − 2 2 Ry = 4 3 Ry , where Ry = 1 3 . 6 eV is the well-known ionization energy of the electron in hydrogen in its ground state. The work function of the metal, then, is ϕ = E γ − K 0 = 1 0 . 2 eV − 8 . 8 eV = 1 . 4 eV .