Isopropanol Reaction Scheme
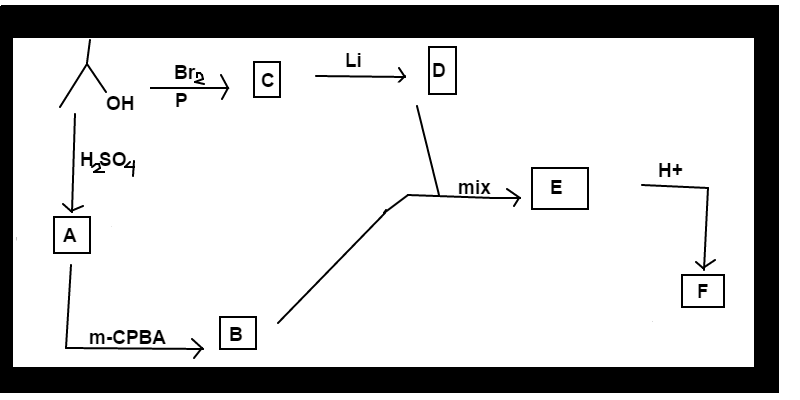 Follow the reaction scheme for isopropanol shown above. For product
E
, choose the best description from each list.
List 1: Substituent Level
1
:
P
r
i
m
a
r
y
:
1
∘
2
S
e
c
o
n
d
a
r
y
:
2
∘
3
T
e
r
t
i
a
r
y
:
3
∘
4
A
l
p
h
a
:
α
5
B
e
t
a
:
β
List 2: Substituent Type
1
:
A
l
k
o
x
i
d
e
2
:
A
l
c
o
h
o
l
3
:
E
p
o
x
i
d
e
4
:
C
a
r
b
o
x
y
l
i
c
A
c
i
d
5
:
E
t
h
e
r
Your answer will be the combo of the boxed digits associated with the correct labels in both lists; for example, if product
E
is a tertiary carboxylic acid, then your answer will be
3
4
Suggested wiki:
Grignard Reagents
Follow the reaction scheme for isopropanol shown above. For product
E
, choose the best description from each list.
List 1: Substituent Level
1
:
P
r
i
m
a
r
y
:
1
∘
2
S
e
c
o
n
d
a
r
y
:
2
∘
3
T
e
r
t
i
a
r
y
:
3
∘
4
A
l
p
h
a
:
α
5
B
e
t
a
:
β
List 2: Substituent Type
1
:
A
l
k
o
x
i
d
e
2
:
A
l
c
o
h
o
l
3
:
E
p
o
x
i
d
e
4
:
C
a
r
b
o
x
y
l
i
c
A
c
i
d
5
:
E
t
h
e
r
Your answer will be the combo of the boxed digits associated with the correct labels in both lists; for example, if product
E
is a tertiary carboxylic acid, then your answer will be
3
4
Suggested wiki:
Grignard Reagents
David's Organic Chemistry Set
David's Physical Chemistry Set
The answer is 21.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
1 solution
Keep posting +1
Relevant wiki: Grignard Reagent