It's come undone
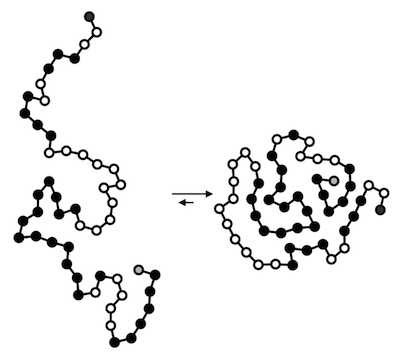 Proteins are molecules responsible for catalyzing chemical reactions, regulating gene expression, sensing changes in the extracellular environment, giving superstructure to the genome, and many other important tasks. They take the form of long chains that fold into compact structures by seeking out shapes that minimize the energy of self-interaction.
Proteins are molecules responsible for catalyzing chemical reactions, regulating gene expression, sensing changes in the extracellular environment, giving superstructure to the genome, and many other important tasks. They take the form of long chains that fold into compact structures by seeking out shapes that minimize the energy of self-interaction.
Consider a simple protein that has two states, completely folded, and completely unfolded, which have a free energy gap of kJ/mole .
You have an ensemble of several million copies of this protein dissolved in buffer. At temperature C, what fraction of the proteins are folded?
Note: You may wish to read about partition functions .
The answer is 0.81.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The equilibrium constant K is given by the formula Δ G = − R T l n K which can be re-arranged to give K = e − R T Δ G .
Substituting in Δ G = 3 7 4 0 J m o l − 1 , R = 8 . 3 1 4 5 J K − 1 m o l − 1 and T = 3 7 + 2 7 3 . 1 5 = 3 1 0 . 1 5 K yields an answer of:
K = 0 . 2 3 4 4 9 5 3 6 0 5 .
Therefore: Y X = 0 . 2 3 4 4 9 5 3 6 0 5 where X = proportion of folded proteins and Y = proportion of unfolded proteins.
We can also say that X + Y = 1 since the total fraction must be 1.
Substituting in X = 1 − Y yields Y 1 − Y = 0 . 2 3 4 4 9 5 3 6 0 5 .
Re-arranging and solving gives Y = 0 . 1 8 9 9 5 2 4 0 3 2 .
Therefore, X = 1 − 0 . 1 8 9 9 5 2 4 0 3 2 = 0 . 8 1 0 0 4 7 5 9 6 8 .