IUPAC nomenclature
Chemistry
Level
2
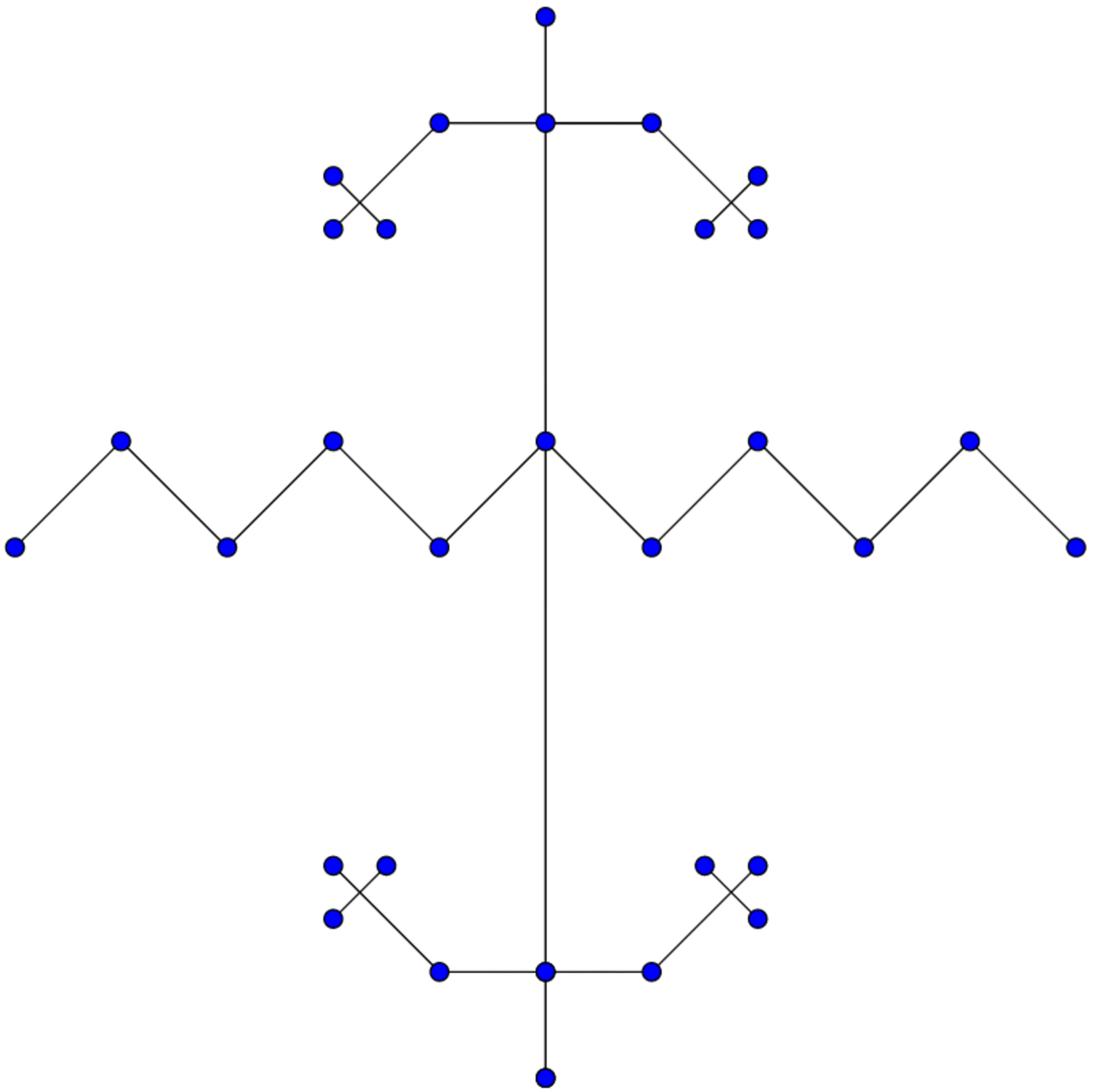
What is the IUPAC name of the hydrocarbon shown above?
Clarification:- The blue circle indicates carbon.
(6,6,2,2) hydroxymethyl undecane
((6,6)-bis ((1,1)-bis ((2,2)-dimethyl propyl)ethyl)) undecane
((5,5)-quad ((2,1)-bis ((2,3)-dimethyl ethyl)propyl)) decane
((1,1)-bis ((6,2)-bis ((3,2)-dicarbonide butyl)methyl)) undecane
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
First of all select select the long carbon chain which is obviously the straight carbon chain of 11 carbons thus giving it the root word undecane. Now, go to the first substituent. There are two identical substituents on the same sixth carbon of the parent chain. So, there positions would get the prefix 6,6-bis (note that the word 'di' is not used since the substituent is complex). Again, the substituent itself has 2 carbons. So, it gets the root word "ethyl". (The common point is not counted as a part of the substituent). Now, there is another pair of identical substituents on this substituent at the first carbon itself, so their positions will be named 1,1-bis. They contain 3 carbons each so they obtain the root word propyl. Again, this substituent has two identical substituents at the same second position, so they get the positon name as 2,2-bis. Since, they contain one carbons only, they get the root word methyl. Now, lets combine these words. Note that the innermost substituents are simple and thus will be prefixed by "di" instead of "bis".
First go for the positon of the other most substituent , then the most of the next substituent, then that of the innermost substituent. After giving the positions, the root words are arranged in the order of innermost to outermost substituents. And in the end place the root word of the long chain.
So, our answer is ((6,6)-bis ((1,1)-bis ((2,2) dimethyl)propyl)ethyl) undecane.
Take care of the paentheses. While we name only for one substituent, the parentheses make them valid for the others too.