A chemistry problem by Koushik Vennelakanti
Chemistry
Level
2
Find the maximum number of electrons that can fit in a 4f orbital in a uranium like species?
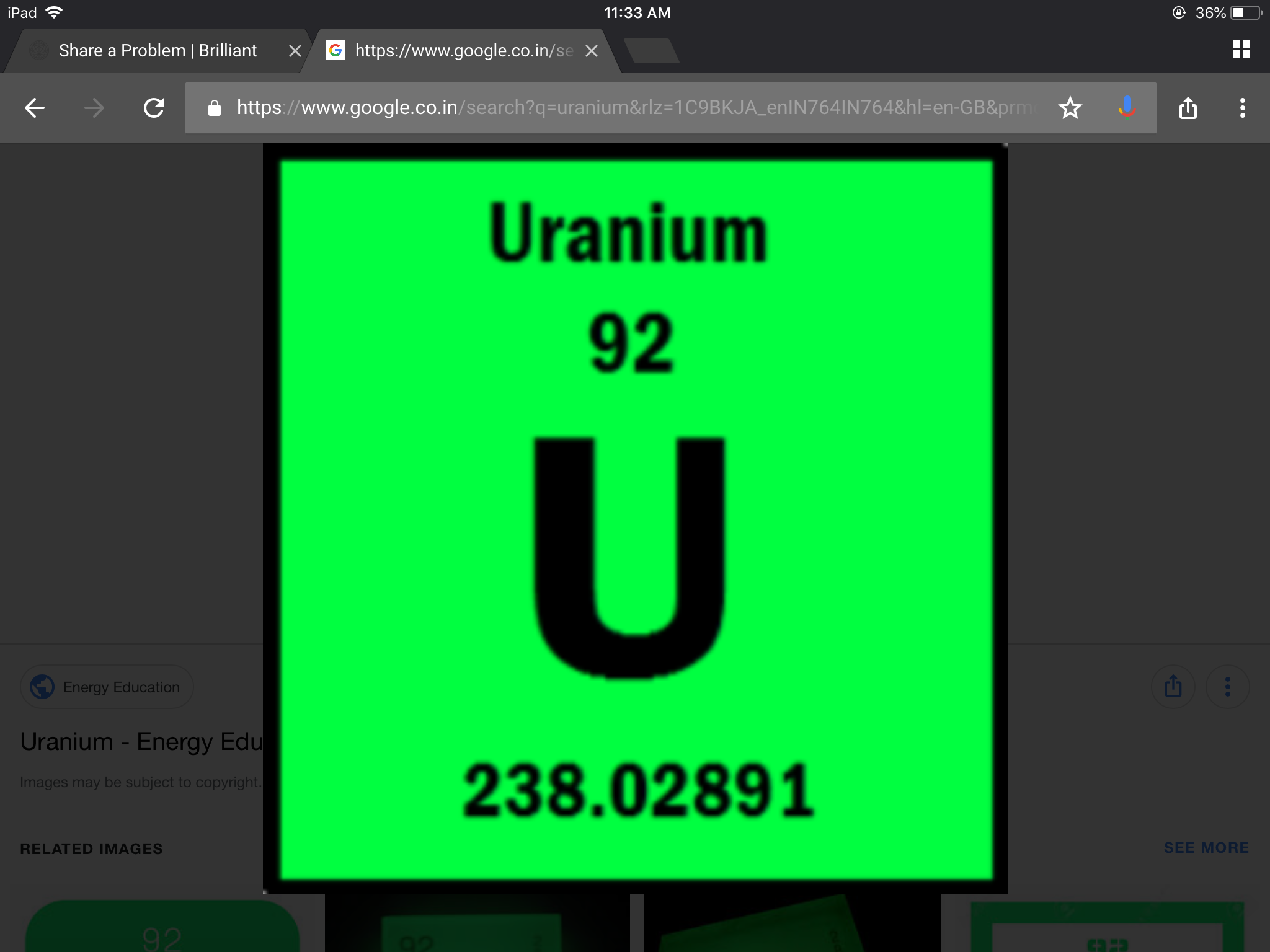
0
7
14
2
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
How much ever big the electronic configuration of a element (which have 4 f sub shell filled) the maximum number of electrons Ina orbital cannot exceed 2. As orbital is the one box which can accommodate 2 electrons.