Let's Begin the Craze for Inorganic
Now I am gonna post a inorganic blast off Series. I see that my questions are encouraged by all of you so that's increasing my desire to make more new original questions. So here is the first question (Starting with a simpler one). Let's see who will post the solution first.
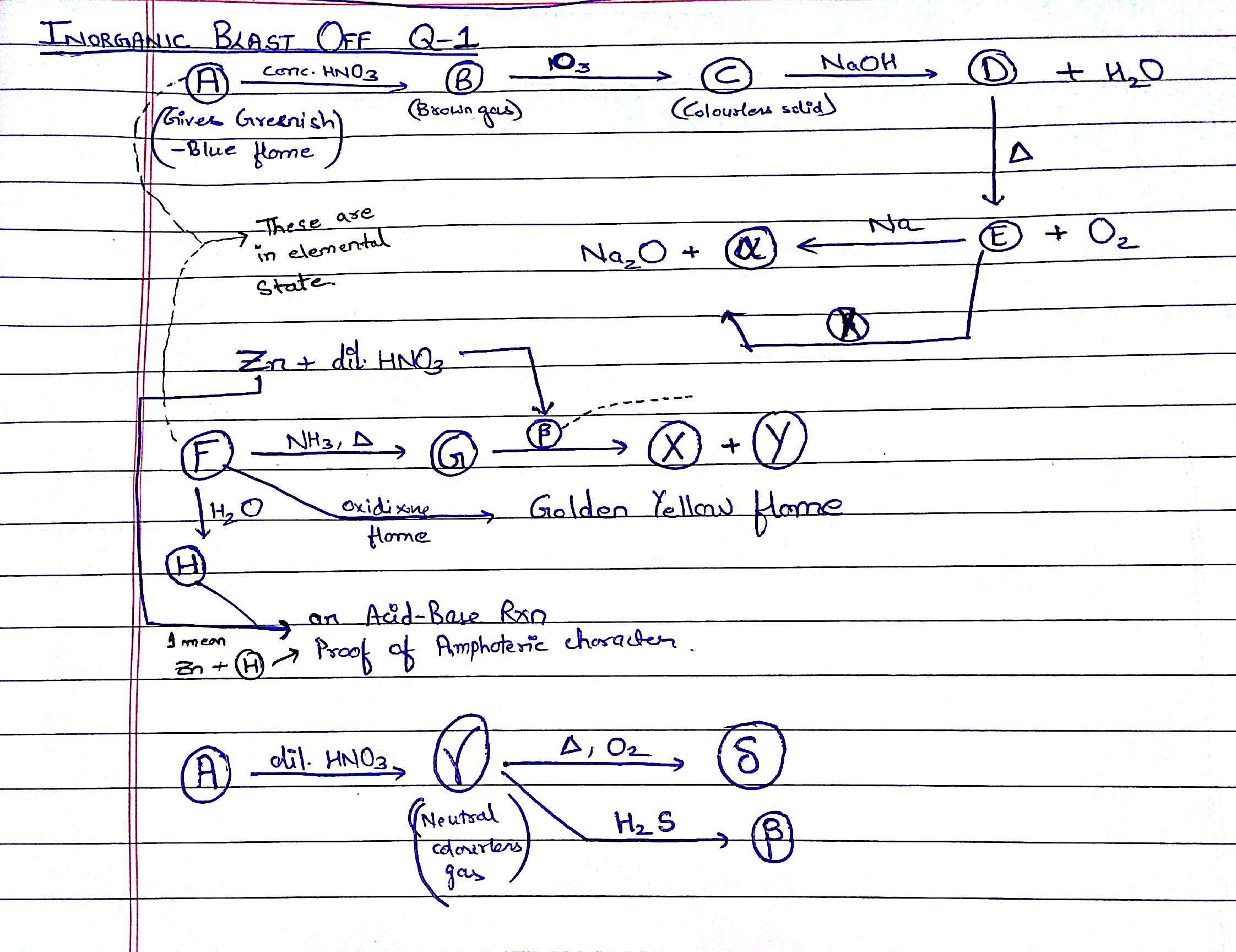
That is X which is added to E!
Enter your answer as .
Where represents molecular weight of X.
This is a part of my set Inorganic Fun
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
A = C u
B = N O 2
C = N 2 O 5
D = N a N O 3
E = N a N O 2
F = N a
G = N a + ( a m ) + e − ( a m )
H = N a O H
X = N a N H 2
Y = H 2
α = N 2
β = N 2 O
γ = N O
δ = N O 2 .
4 Z n ( s ) + 1 0 H N O 3 ( a q ) ⟶ N H 4 N O 3 ( a q ) + 4 Z n ( N O 3 ) 2 ( a q ) + 3 H 2 O ( l )