Linear chain
Which electrostatic work must be applied so that a cation can be removed from this linear chain of ions? Caculate the value in units of electronvolts (eV) and round it to the nearest integer.
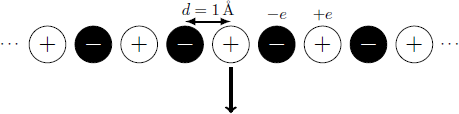
The distance between two neighboring ions is the charge values are equal to the elemental charge .
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The electrostatic energy is a sum over all ions in the chain at positions x j = j ⋅ d and charge q j = ( − 1 ) j ⋅ e : W = 4 π ε 0 1 j = 0 ∑ ∣ x 0 − x j ∣ q 0 q j = 4 π ε 0 d e 2 j = 0 ∑ ∣ j ∣ ( − 1 ) j = − 4 π ε 0 d e 2 ⋅ 2 j = 1 ∑ ∞ j ( − 1 ) j + 1 = − 2 π ε 0 d e 2 ln 2 = 3 . 1 9 8 ⋅ 1 0 − 1 8 J = 1 9 . 9 6 eV ≈ 2 0 eV With the help of the logarithm series lo g ( 1 + x ) = ∑ j = 1 ∞ j ( − 1 ) j ⋅ x j .