Molar Heat capacity
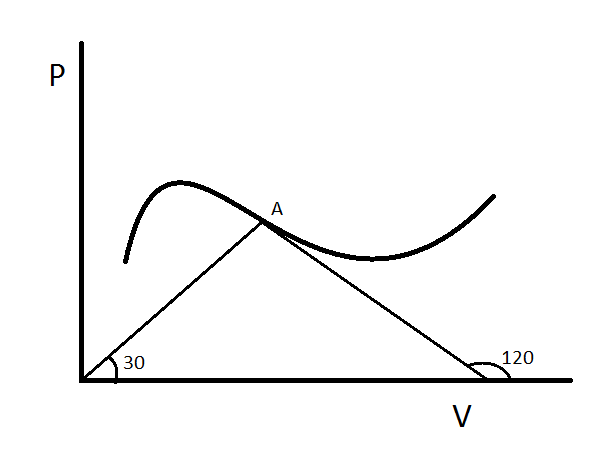
Mole of an Ideal monoatomic gas undergoes a process depicted in the figure. At some point A of the diagram line joining the A to origin makes an angle degrees with the axis and the tangent at point A makes an angle degrees with the axis.
Find the instantaneous molar heat capacity of gas at point A in Take
The answer is 2.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Easy problem ! F r o m g r a p h , P = 3 V . . . ( 1 ) a n d d V d P = − 3 . . ( 2 ) S i n c e P V = n R T P d V + V d P = n R d T N o w u s i n g 1 a n d 2 a n d s o m e s i m p l e m a i n p u l a t i o n , P d V = 2 − n R d T d U = 2 3 n R d T d q = d U + d W d q = n R d T M o l a r h e a t c a p a c i t y i s R