Move beyond mains
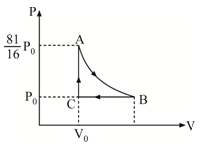
A triatomic non-linear ideal gas is taken through a cyclic process as shown. The process is an adiabatic process. Find percentage efficiency of the cycle to the nearest integer.
Note: Vibrational degrees of freedom are neglected.
The answer is 22.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Let the gas expand n times in the process.(T1 temp at B,T0 temp at C).gamma=y= C v C p
Q2(Heat released)=CpXT1X(1- n 1 )
Since adiabatic,T0=T1Xn^(y-1).
So Q1=CvX(n^y-1)X n T 1
E=1- Q 1 Q 2 =1- n y − 1 y ( n − 1 )
Calculate with values,to get n= 8 2 7 and y= 3 4
EX100=22(approx.)=ANSWER