Move the piston, you don't want it to burn
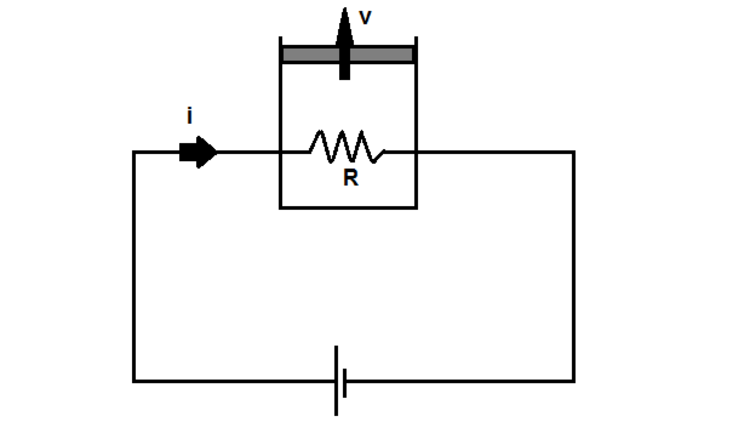 A resistor of resistance
connected to an external battery is placed inside a cylinder fitted with a friction-less piston of mass
, area of cross section
and contains
moles of an ideal mono-atomic gas. A current
flows through the resistance.
A resistor of resistance
connected to an external battery is placed inside a cylinder fitted with a friction-less piston of mass
, area of cross section
and contains
moles of an ideal mono-atomic gas. A current
flows through the resistance.
The temperature of the gas is maintained constant by moving the piston with a velocity upward (measured in ).
What is the value of ?
Details and Assumptions
- The cylinder is thermally insulated.
Try my set for more problems.
The answer is 225.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
@Vishwak Srinivasan i have found a error in question that we have
-temperature constant(provided in question)
-pressure constant as the piston moving with constant velocity
though all the parameters of ideal gas equation is fixed volume is changing. if i am wrong somewhere please correct me i would like to know where i went wrong.