Naming crown ethers
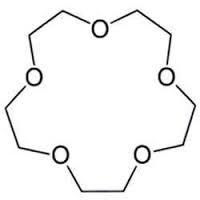 The molecule above is a crown ether. They are named as
where
is the total number of atoms (except hydrogen) present in the ring and
is the number of oxygen atoms. For the above crown ether, what is the value of
?
The molecule above is a crown ether. They are named as
where
is the total number of atoms (except hydrogen) present in the ring and
is the number of oxygen atoms. For the above crown ether, what is the value of
?
The answer is 20.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
For the above crown ether the total no. of carbon atoms = 1 0 and total no of oxygen atoms = 5 .Hence n = 1 0 + 5 and m = 5 so the name of the compound is 1 5 − c r o w n − 5