Nuclear transmutation process
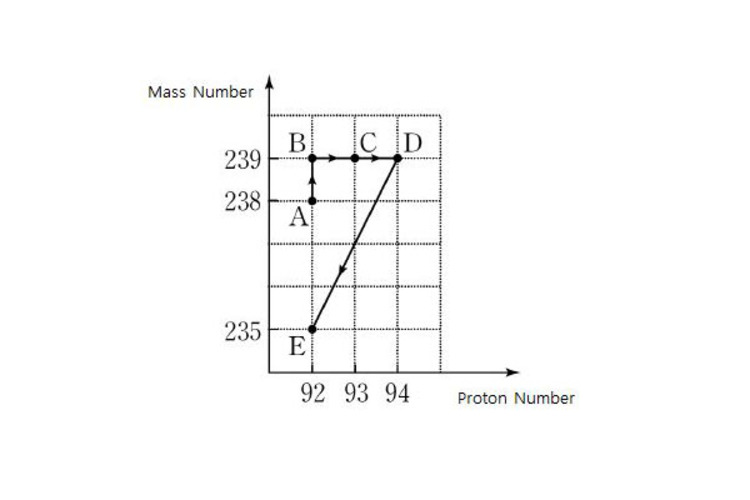 The above diagram shows the changes in the mass number and number of proton when an atomic nucleus
becomes
by the nuclear transmutation process
Which of the following is true?
The above diagram shows the changes in the mass number and number of proton when an atomic nucleus
becomes
by the nuclear transmutation process
Which of the following is true?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Process A → B does not increase in mass number but atomic number by 1 indicates addition of an electron. Increase in mass numbers by 2 in B → C → D indicates that 2 neutrons are added. Finally, the drop in mass number by 2 in D → E indicates 2 n e u t r o n s a r e e m i t t e d .