Of Alcohols and Heavy Water
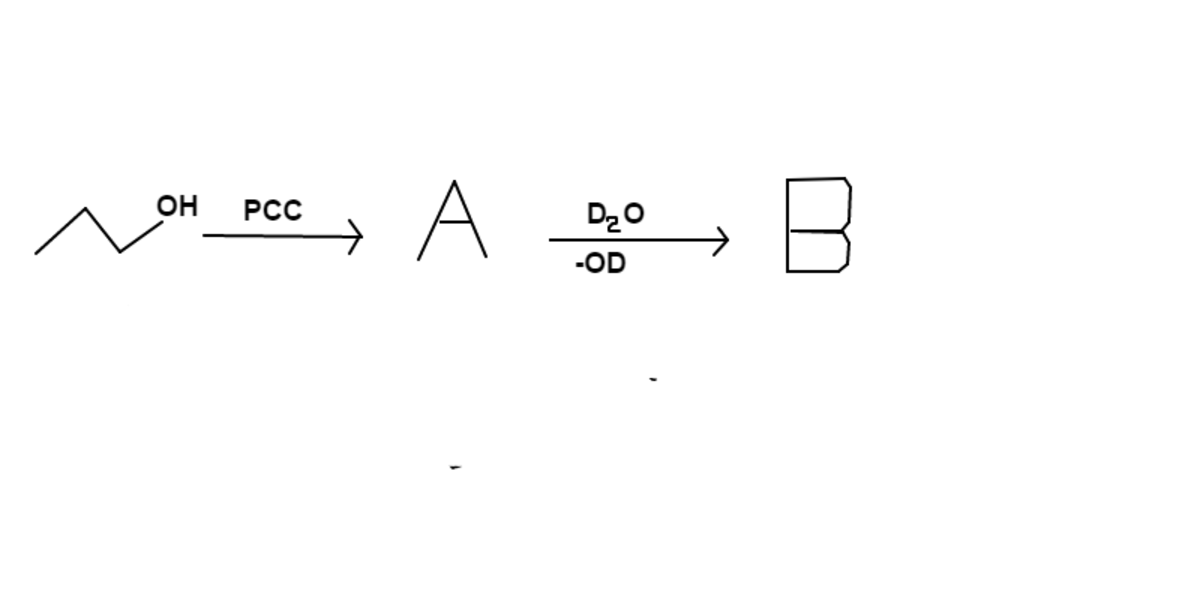
1-propanol is reacted with pyridinium chlorochromate ( ) to form . is then transferred to a solution of heavy water ( ) that also contains deuterated hydroxide ( ) to form .
Give your answer as the number of hydrogens ( ) in , divided by the number of deuteriums ( ).
Give your answer to 2 significant figures.
The end product, , is not the result of aldol condensation.
David's Organic Chemistry Set
David's Physical Chemistry Set
The answer is 2.0.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
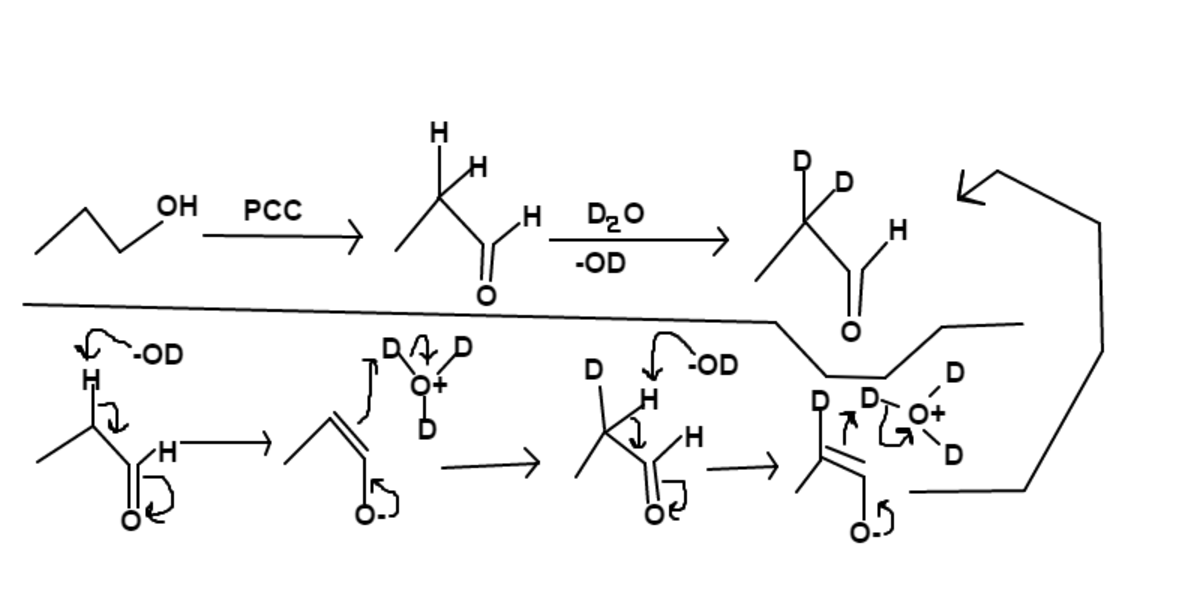 The hydrogens on the carbon
a
l
p
h
a
to the carbonyl carbon of propanal are very acidic; thus they can be pulled off by
−
O
D
and replaced with deuteriums from
D
3
O
+
B
has
4
H
and
2
D
; therefore
A
n
s
w
e
r
→
2
D
4
H
=
2
.
0
The hydrogens on the carbon
a
l
p
h
a
to the carbonyl carbon of propanal are very acidic; thus they can be pulled off by
−
O
D
and replaced with deuteriums from
D
3
O
+
B
has
4
H
and
2
D
; therefore
A
n
s
w
e
r
→
2
D
4
H
=
2
.
0