This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
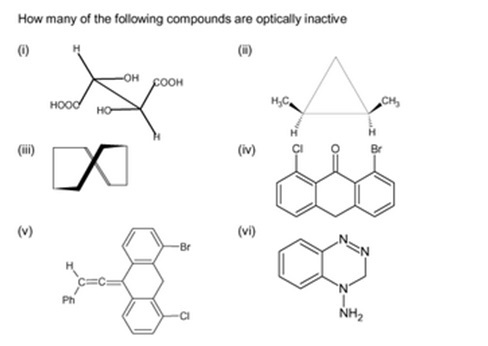
For a molecule to be optically active, it must have an atom with 4 bonds coming off it. This implies iv, v and vi are inactive.
iii Is also inactive since every carbon has 2 Hs coming off it.
i and ii both have 2 chiral carbons, so are optically active.