This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
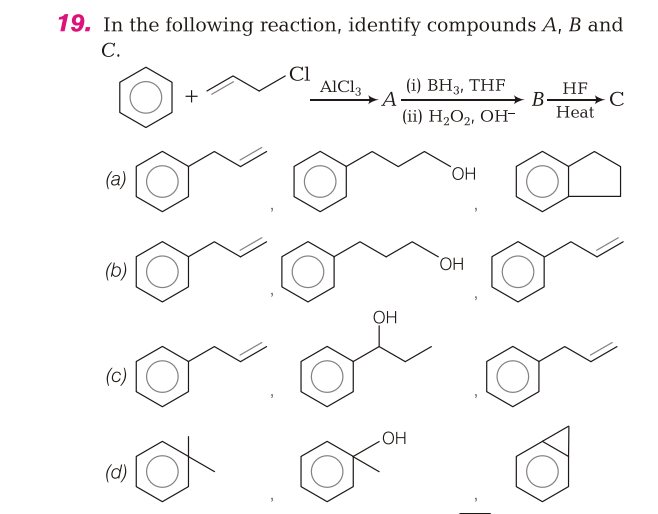
Firstly AlCl3 being a lewis acid will form AlCl4- generating a stable allylic carbocation. Now this carbocation will attack benzene through Electrophilic aromatic substitution (through Arenium ion mechanism) . Now You are doing Hydroboration Oxidation of alkene which follows antimarkovnikov's regioselectivity Hence OH- will attack on that carbon which has more number of hydrogen . Now You are doing acid catalysed dehydration fo alcohol in which again a carbocation will be formed which will again the attack the benzene in the style of Electrophilic aromatic substitution and result in the formation of quite stable (less angle strain) 5 membered cyclic ring! :)
Note-
Rearrangement Does NOT OCCUR in Hydroboration Oxidation as true carbocation does not form.(Proceeds through formation of trialkyl borane followed by its hydrolysis)
Acid catalysed dehydration of alcohol Follows E1 Reaction (Unimolecular Elimination) so true carbocation is formed.
Arenium Ion is a stable Ion which gets stabilised a bit through resonance. Later on it loses a proton to regain aromaticity and follow Huckel's rule. (Arenium ion is Non Aromatic due to non planarity)