Organic Chemistry 2: Advanced Hydrocarbon Formation
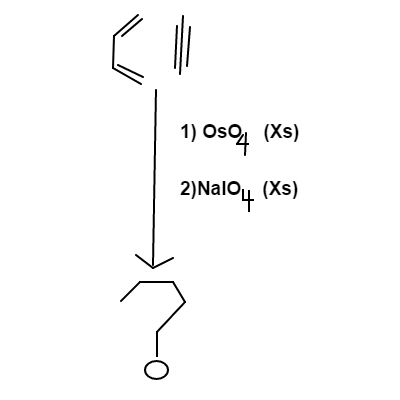 A mixture of butadiene and ethyne is treated first with osmium tetroxide and then with sodium periodate, with both reagents being in excess (
X
s
).
A mixture of butadiene and ethyne is treated first with osmium tetroxide and then with sodium periodate, with both reagents being in excess (
X
s
).
Your answer will be a three digit number that satisfies the following conditions:
(a) : The first digit will be the number of oxygens in the final product(s).
(b) : The second digit will be the number of carbons in the final product(s).
(c) : The third digit will be the number of hydrogens in the final product(s).
For example, If the final product is C X 6 H X 8 O X 1 , then your answer will be 168.
David's Organic Chemistry Set
David's Physical Chemistry Set
The answer is 234.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
1 solution
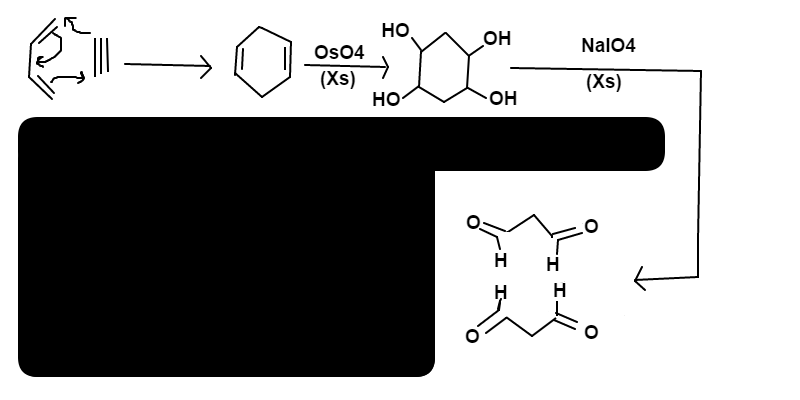 The final product is
propadial
(
C
3
H
4
O
2
)
Answer
=
2
3
4
The final product is
propadial
(
C
3
H
4
O
2
)
Answer
=
2
3
4
Exactly :)
Nice questions !! KEEP ON posting:)
nice one !
@David Hontz Is the reaction between butadiene and ethyne spontaneous?? Cause, in the solution provided, they are made to react first.
Log in to reply
Energetically, 3-pi bonds are broken in the reactants and formation of the 2 sigma-bonds + 1 pi-bond in the product has a negative enthalpy value, therefore the reaction is exothermic and spontaneous. This particular reaction is called D i e l s − A l d e r R e a c t i o n , with an alkyne being used in place of an alkene, and is enthalpically driven. To answer your question; yes, butadiene and ethyne do react spontaneously, which makes these kinds of reactions very fun to work with!
Nice one dude
Diels alder reaction needs heating