Organic Chemistry at its best!
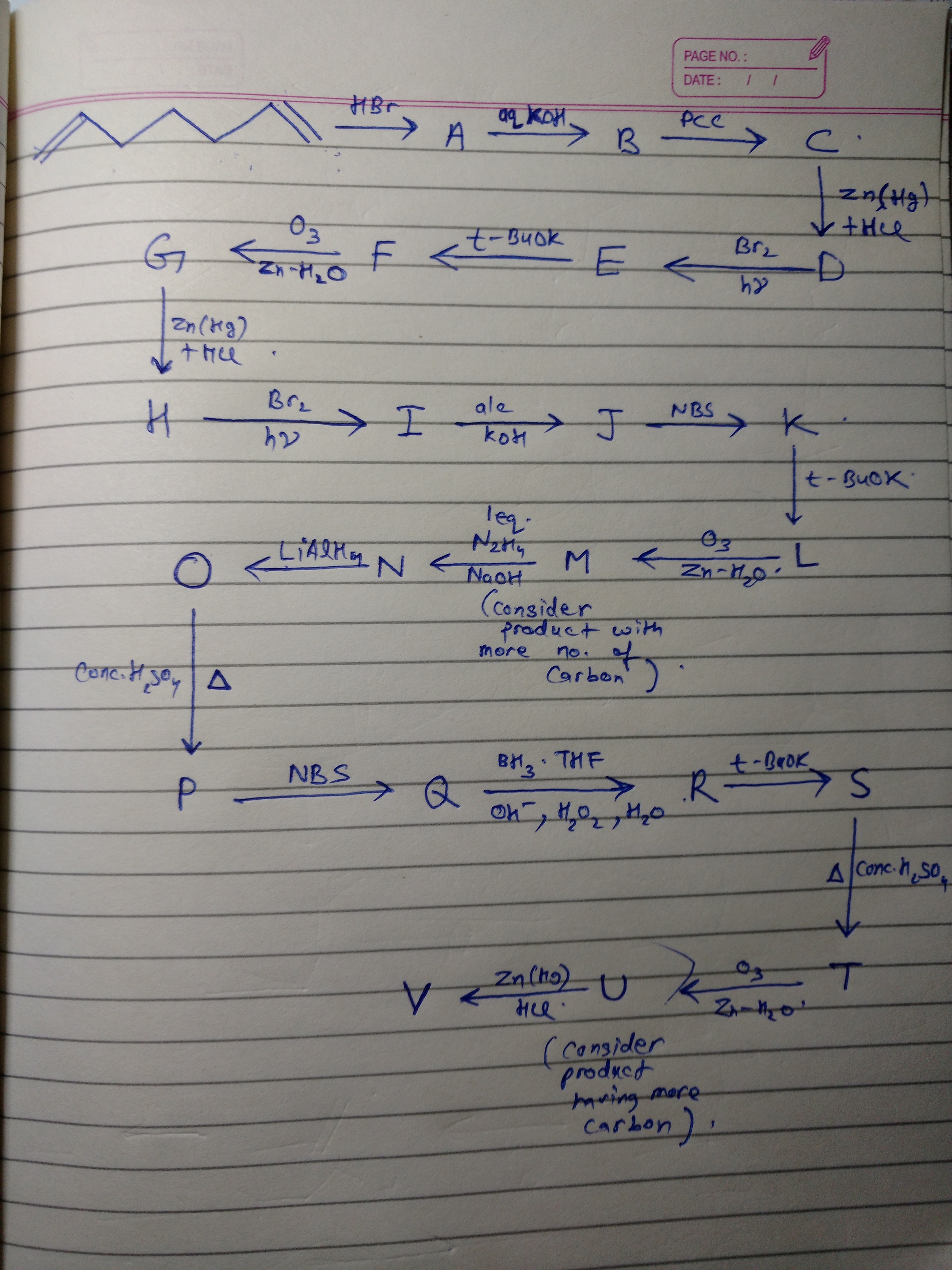
Answer the following questions on the basis of final product.
•Number of carbon atoms be X.
•Degree of unsaturation be Y.
•Number of halogens present be Z.
• Number of oxygens present be A.
•Number of sp3 hybridized carbon be B.
Enter
The answer is 92.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The product formed is cyclopropane. Hints: 1) For the first rxn when H+ attacks a double bond, carbocation is formed which further attacks the other double bond , forming a carbocation and Br- gets attached at that position ( Note a cyclic 6 membered ring is formed) 2) While forming 'T' from 'S' , a special type of carbocation rearrangement takes place which becomes highly stable due to "SIGMA RESONANCE" . (Cyclopropyl methyl carbocation is formed)