Otto-Kurt Extension
An extension to Teleanu Florin's problem .
 (
Pausinystalia johimbe
)
(
Pausinystalia johimbe
)
The final compound is a diastereomer of Yohimban (the base chemical structure of various alkaloids in the Pausinystalia plant genera) and is synthesised using the following scheme:
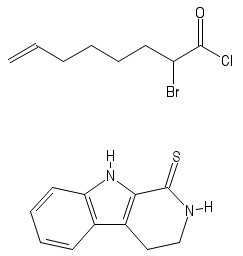
The above compounds are heated in and generate an intermediate A . When A is reduced, a penta-cyclic compound is formed:
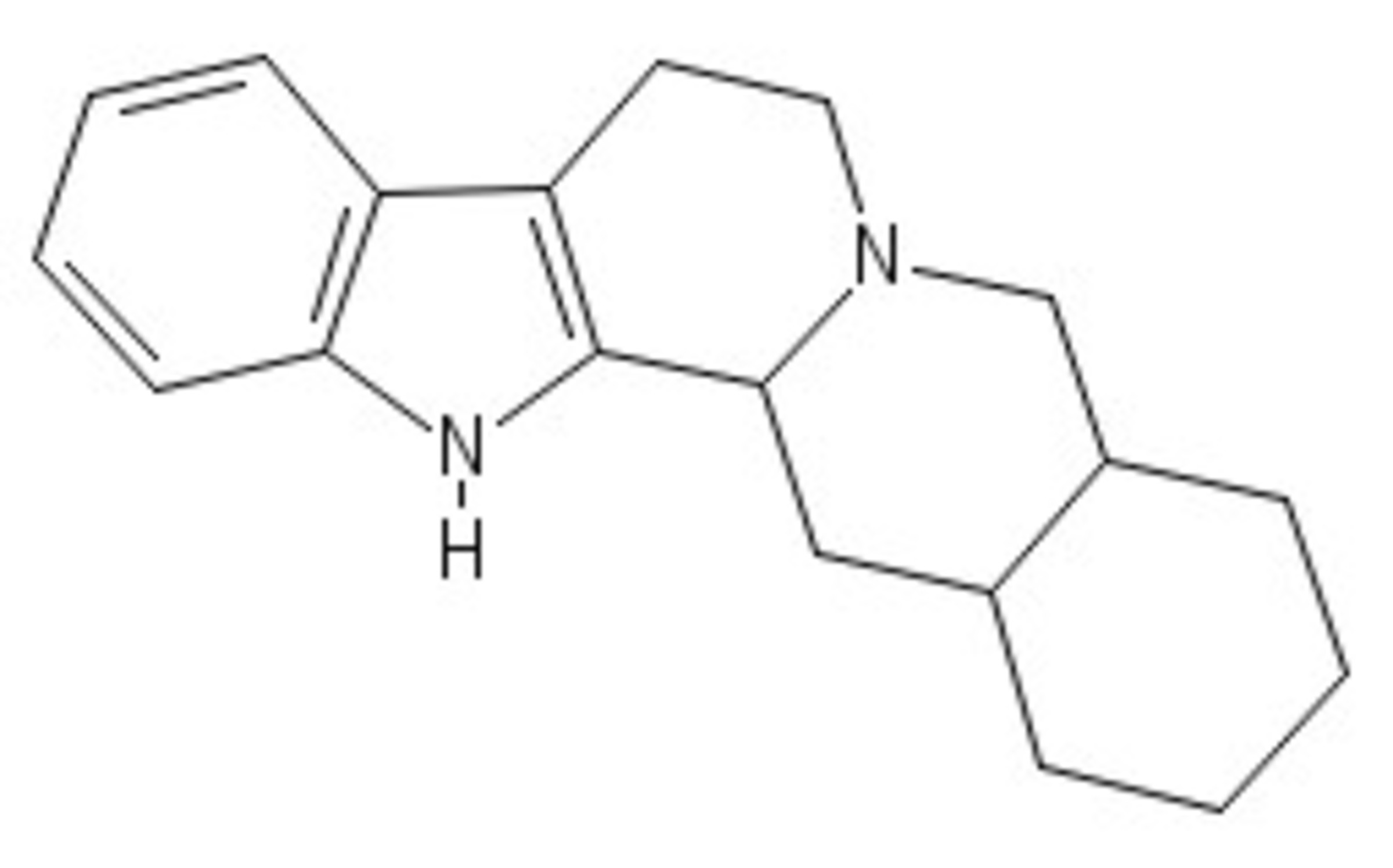
Let be the mass of compound A and be the mass of the final product.
Calculate
BONUS
Determine configuration of all stereo-centres in the final product if the acyl chloride's chiral centre is in the R configuration.
Image credit : Pinterest
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
In this reaction E t 3 N plays a duel role of a solvent and a base. The first step involves the abstraction of the sulfamide hydrogen and the subsequent nucleophillic attack on the acyl chloride. It doesn't attack the bromine atom as the S N 2 route is hindered and S N 1 cannot occur due to orbital interactions.
The next step starts from the realisation that the sulfamide is a hidden nucleophile and can attack the bromine atom in an S N 2 manner due to its close proximity. After the attack E t 3 N abstracts the hydrogen atom.
Now an intermolecular cyclo-addition occurs to generate the intermediate A :
It is clear that β is 280 and from the above structure we see that α is 324. Hence the answer is 4 4
Determining stereochemical configuration is left as an exercise for the reader.