Out of the oven, into the frying pan
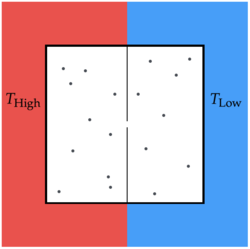 Consider a gas in the device shown above. Two halves of the box are separated by a dividing wall that has a small hole in it, which is just slightly wider than the diameter of the gas molecules. Each half of the box is in contact with a heat bath held at a given temperature.
Consider a gas in the device shown above. Two halves of the box are separated by a dividing wall that has a small hole in it, which is just slightly wider than the diameter of the gas molecules. Each half of the box is in contact with a heat bath held at a given temperature.
If there are gas molecules in the box, find the expected number of gas molecules in the hot side when the box reaches steady state.
Assumptions and Details
The answer is 34833.147735479.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
First we assume that the amounts of molecules in each part are respectively n m 1 and n m 2 , than we have: n m 1 + n m 2 = N / N a where N a is Avogadro's constant.
In the steady state we have following relations: Δ N 1 n m 1 S v 1 t = Δ N 2 = n m 2 S v 2 t
where S is an area of the hole, v 1 and v 2 the average speeds of the molecules in each part, t a small time interval, and Δ N 1 and Δ N 2 are the numbers of molecules which pass through the hole during the interval t .
After calculating this we get a relation: n m 1 T 1 = n m 2 T 2 and therefore: N 1 = N T 1 + T 2 T 2 = 3 4 8 3 3 . 1 4 8 K (using N 1 = n m 1 N a )