This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
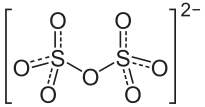
Let the oxidation state, of sulphur be x .
We know that the oxidation state of oxygen is − 2 . Therefore, 2 x + 7 ( − 2 ) = − 2 2 x = − 2 + 1 4 x = 2 1 2 x = + 6