Oxidation State
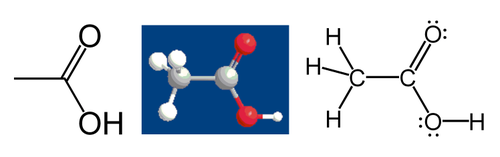 The oxidation state, often called the oxidation number, is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were
% ionic. Oxidation states are typically represented by integers, which can be positive, negative, or zero. What are the respective oxidation states (oxidation numbers) of the underlined atoms below?
The oxidation state, often called the oxidation number, is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were
% ionic. Oxidation states are typically represented by integers, which can be positive, negative, or zero. What are the respective oxidation states (oxidation numbers) of the underlined atoms below?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!