Percentage of Copper in Brass Screw
Hamza conducts an experiment to find how much copper is in a typical brass screw. Hamza thinks about how Beer's law can help find his concentration, but for that he needs a solution.He first weighs his brass screw and obtains a mass of . He then proceeds to dissolve the brass screw with an oxiding agent in the reaction
Hamza then dilutes the Copper Nitrate solution using a
volumetric flask. He measures the solutions' absorbance and obtains an absorbance of
. He sets his calibration curve for
and obtains an equation
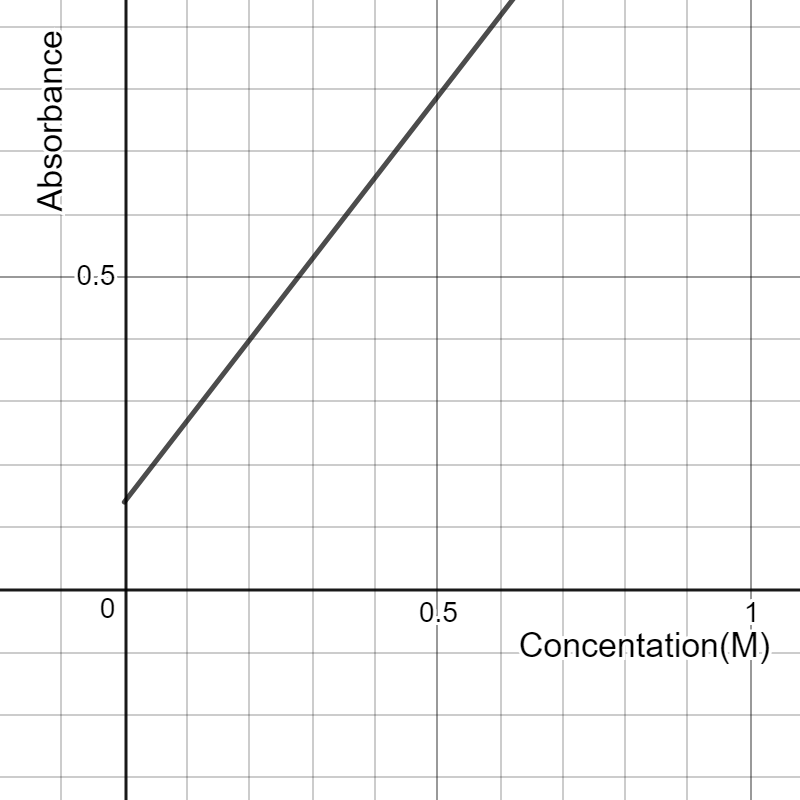
Given the data above, find the percentage of copper in the brass screw.
The answer is 55.1.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!