Photoelectric Effect
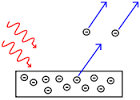
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons.
The work function of an element is the minimum energy needed to remove an electron from the solid element. The work function for rubidium is .
What is the minimum amount of energy needed to eject a single electron from the surface of rubidium metal?
Details and Assumptions:
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
This is mostly a matter of reducing down the given constants. We want to find the energy required for 1 atom.
We're told that the work function for Rubidium is 2 0 8 . 4 k J mol − 1 , so lets find the energy per atom.
2 0 8 . 4 k J mol − 1 = 2 0 8 4 0 0 J mol − 1
We're given (and know Avogadro's number) that 6 . 0 2 2 × 1 0 2 3 a t o m mol − 1
So 6 . 0 2 2 × 1 0 2 3 a t o m mol − 1 2 0 8 4 0 0 J mol − 1 = 3 . 4 6 × 1 0 − 1 9 J a t o m − 1 .