Preparing an indicator.
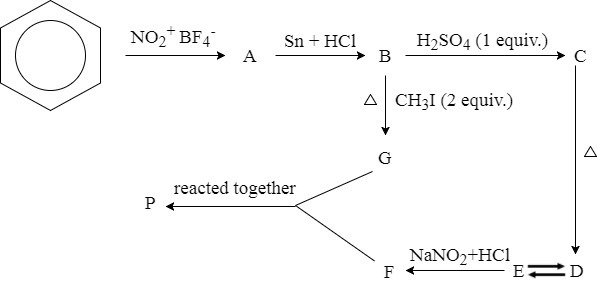
In the above-shown step by step synthesis of product , is a zwitterion of product which is prepared by heating at . The product is an indicator which shows the colour change at which range?
Note: Select the closest and most appropriate range from the options given. Data values may differ slightly from place to place.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
First I looked up a list of acid-base indicators having the desired colour change range. Candidates are :
The process indicates that the synthesis involves the combination of two or more aromatic rings. In some more detail:
Product A is the product of benzene and nitronium tetrafluoroborate. It is probably benzene nitrate ( C 6 H 5 N O 2 ), although that is usually synthesized using sulphuric acid and nitric acid.
Product A reacts with tin and HCl to form product B, which then must be aniline ( C 6 H 5 N H 2 )
Product G then is dimethylaniline ( C 6 H 5 N ( C H 3 ) 2 ), since the iodomethane will substitute the hydrogens with methyl groups.
I did not look at the other branch C-D-E-F too well, but it looks like some sulphuric group is added to the ring, and the product might very well be C 6 H 7 N O 3 S (sulfanilic acid).
Looking at the molecule structure of methyl orange we recognise both aromatic molecules that constitute it. So I went for the 2nd answer.