Probable Bohr's model
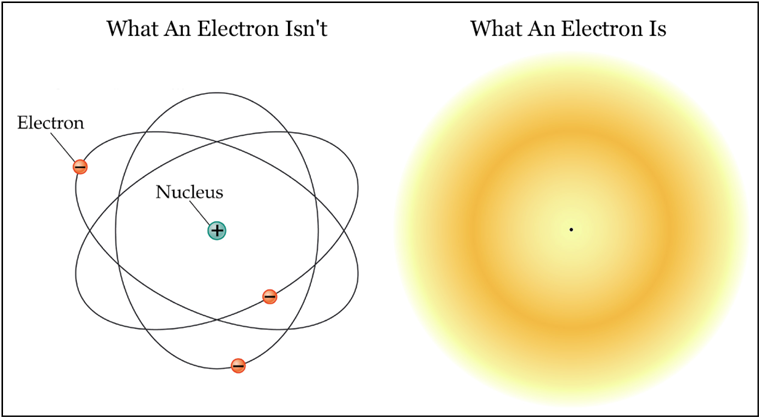
Instead of thinking about the electrons as tiny balls orbiting the nucleus, the modern understanding is that their positions are smeared out throughout the atom. However, we could only find them only at one point when we do a measurement.
The function that gives the probability of an electron being detected at a particular point is called the probability density function.
Given the probability density as a function of the radius of the Hydrogen atom in its non-excited state is
Calculate the probability that the electron will be found outside the Bohr's radius.
-
indicates the Bohr's radius.
-
Round your answer to decimal places
The answer is 0.677.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The probability is found by integrating the radial probability density from Bohr's radius to infinity.
P = ∫ a 0 ∞ P 1 s ( r ) d r = a 0 3 4 ∫ a 0 ∞ r 2 e a 0 − 2 r d r
Now put z = a 0 2 r . z = 2 when r = a 0 and d r = 2 a 0 d z
P = 2 1 ∫ 2 ∞ z 2 e − z d z = − 2 1 ( z 2 + 2 z + 2 ) e − z ) ∣ 2 ∞
P = 5 e − 2 = 0 . 6 7 7