This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
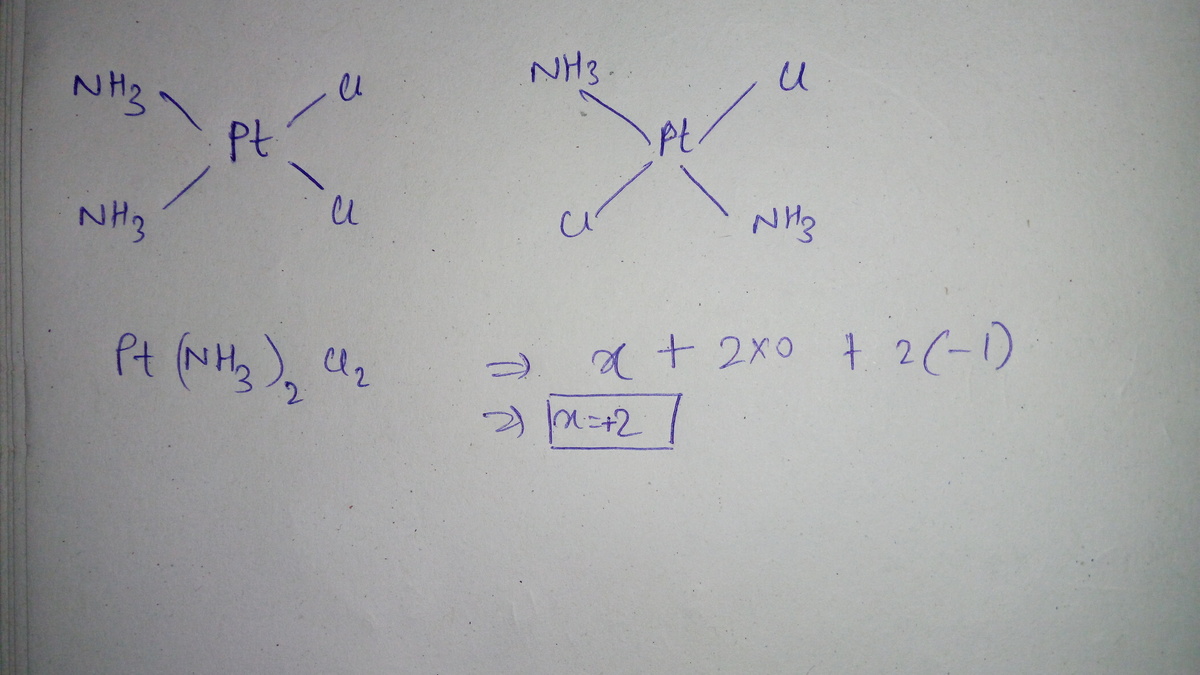
it is a complex in which the ammonia is a neutral ligand and Cl is anionic ligand . so in this compound there are 2 chloride ions as the complex is neural charge of platinum must be +2