radioisotopes!
Chemistry
Level
2
what is the most stable radioisotope of hydrogen?
(view the solution after completing the question, the answer is further explained.)
Hydrogen 6
Protium
Hydrogen 7
hydrogen 5
tritium
deuterium
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Tritium is the most stable radioactive isotope of hydrogen, deuterium is a non-radioactive isotope of hydrogen and Protium is just plain old hydrogen. from here it gets interesting hydrogen 4, 5,6 and 7 decay in a matter of yoctoseconds .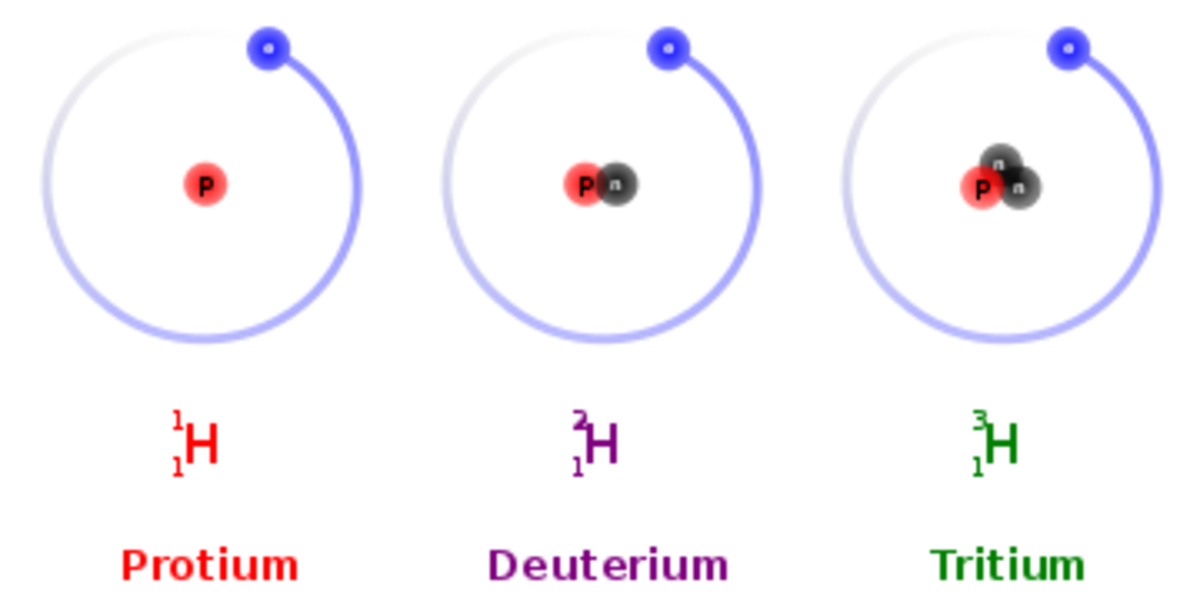
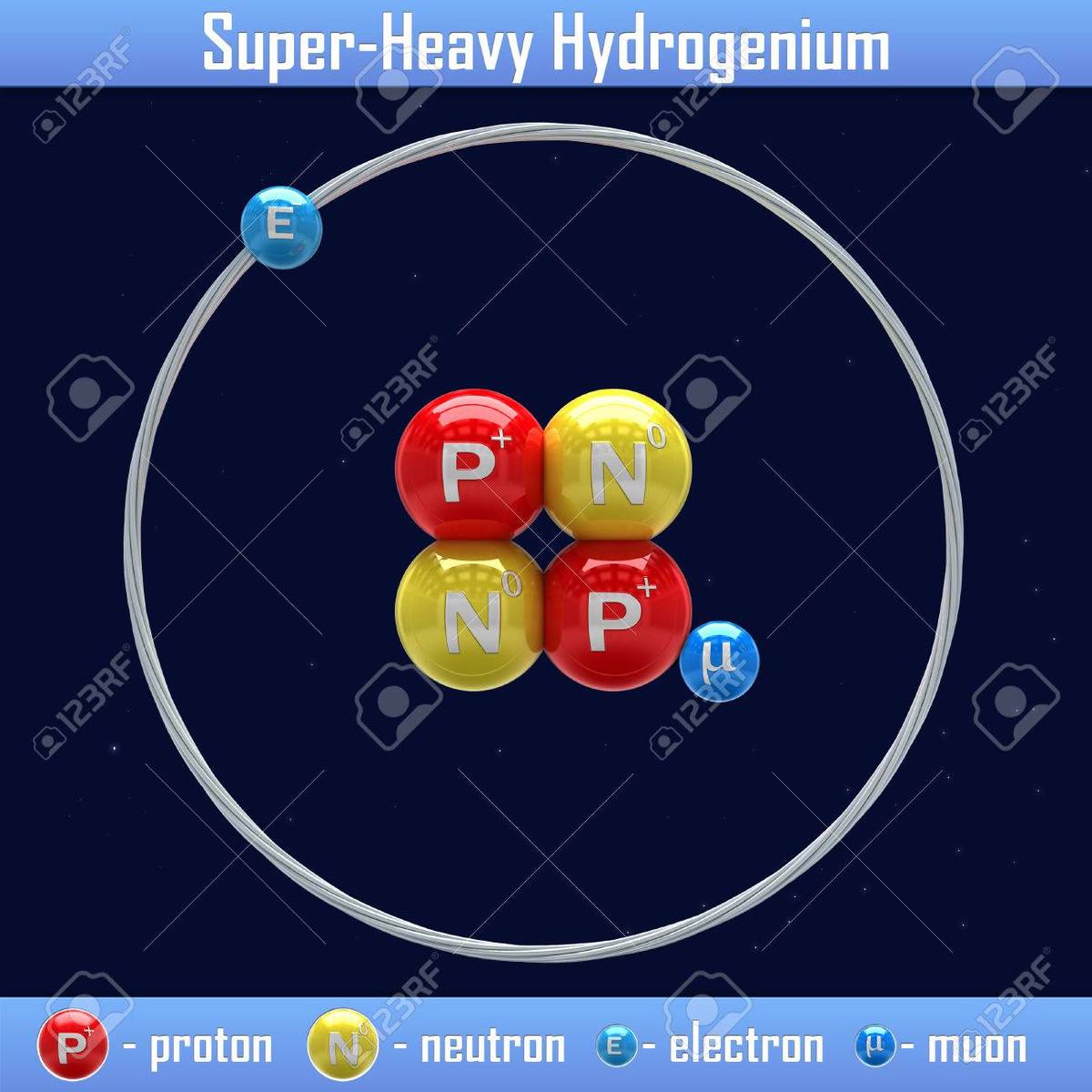
There is also one more isotope of hydrogen known as Hydrogen-4.1, which also known as "neutral muonic helium", it is called that because it is similar to helium as it has 2 protons and 2 neutrons, but one of its electrons is replaced by a muon. Since the orbital of the muon is very near the atomic nucleus, that muon can be seen as a part of the nucleus, so the whole atom can be described as: The atomic nucleus is composed of 1 muon, 2 protons and 2 neutrons, and only one electron orbiting, so it can be considered as an exotic isotope of hydrogen. !