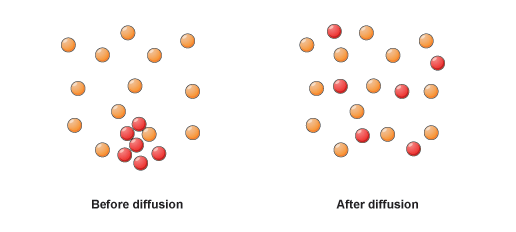
Rate of Diffusion.
 A gas
has a molar mass of 144 g/mol and a diffusion rate of
Another gas
has a molar mass of 36 g/mol and a diffusion rate of
Find the value of
A gas
has a molar mass of 144 g/mol and a diffusion rate of
Another gas
has a molar mass of 36 g/mol and a diffusion rate of
Find the value of
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Basic law of Rate of Diffusion inversely proportional to root of its molar mass.
D α 1 / M
D 1 / D 2 = M 2 / M 1
D 1 / D 2 = 3 6 / 1 4 4
D 1 / D 2 = 6 / 1 2
D 1 / D 2 + 1 = ( 1 / 2 ) + 1 = 1 . 5