Reactivity of alkali metals
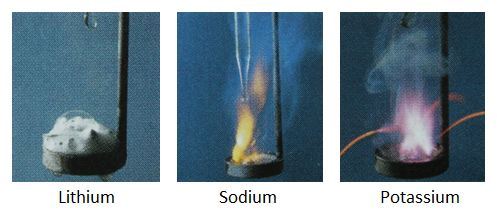 The alkali metals are a group in the periodic table consisting of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).This group lies in the s-block of the periodic table as all alkali metals have their outermost electron in an s-orbital. Because of their high reactivity, they must be stored under oil to prevent reaction with air, and are found naturally only in salts and never as the free element. But the reactivities of alkali metals are not the same, instead sodium is more reactive than lithium and potassium is more and more reactive than sodium, as shown above. Which of following can explain these differences in reactivities between alkali metals?
The alkali metals are a group in the periodic table consisting of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).This group lies in the s-block of the periodic table as all alkali metals have their outermost electron in an s-orbital. Because of their high reactivity, they must be stored under oil to prevent reaction with air, and are found naturally only in salts and never as the free element. But the reactivities of alkali metals are not the same, instead sodium is more reactive than lithium and potassium is more and more reactive than sodium, as shown above. Which of following can explain these differences in reactivities between alkali metals?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!