Science in space suit
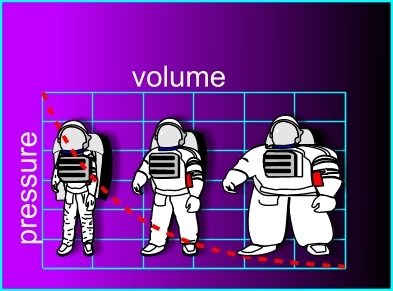 Space suits are designed to be worn in space. A space suit of course must contain a breathable atmosphere with enough oxygen to allow a working astronaut to breathe comfortably. The problem is that in space the external pressure is zero, and the spacesuit attempts to expand like an enormous balloon placing huge stress on the seams and joints of the suit. To make the spacesuits both safe and comfortable, a pressure compromise is used. The nitrogen is removed from the air supply within the suit so that only the oxygen remains. This reduces the internal pressure of the suit by about
%. This causes the spacesuit to undergo slight pressure expansion, but much less so than if it were filled with air at standard atmospheric pressure. Which of the following can explain this phenomenon?
Space suits are designed to be worn in space. A space suit of course must contain a breathable atmosphere with enough oxygen to allow a working astronaut to breathe comfortably. The problem is that in space the external pressure is zero, and the spacesuit attempts to expand like an enormous balloon placing huge stress on the seams and joints of the suit. To make the spacesuits both safe and comfortable, a pressure compromise is used. The nitrogen is removed from the air supply within the suit so that only the oxygen remains. This reduces the internal pressure of the suit by about
%. This causes the spacesuit to undergo slight pressure expansion, but much less so than if it were filled with air at standard atmospheric pressure. Which of the following can explain this phenomenon?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
PRESSURE is inversely proportional to VOLUME