A Chloro-ether-diene Reaction
Chemistry
Level
3
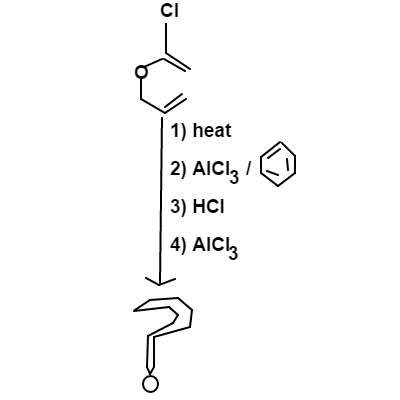
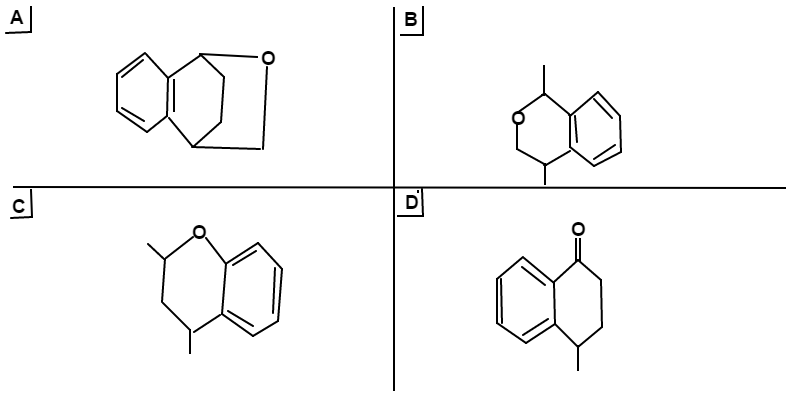
For the above series of reactions, choose which option below represents the major product.
David's Organic Chemistry Set
David's Physical Chemistry Set
None
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Firstly, heat will cause a C l a i s e n r e a r r a n g e m e n t to occur.
F r i e d e l − C r a f t s A c y l a t i o n occurs next when B e n z e n e and A l C l 3 are added
H y d r o c h l o r i n a t i o n occurs on the terminal double bond; this will follow M a r k o v n i k o v X ′ s r u l e .
Lastly, the re-addition of A l C l 3 causes F r i e d e l − C r a f t s A l k y l a t i o n to occur. The cation formed will be isolated, thus rearrangements will not occur before joining the benzene ring.
A n s w e r → D