Sigma and Pi Bonds
Chemistry
Level
1
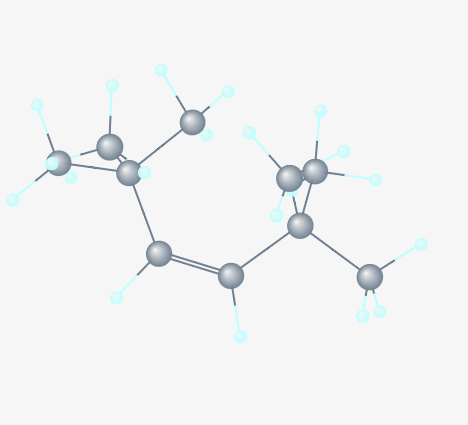 2,2,5,5-tetramethyl-3-hexene
2,2,5,5-tetramethyl-3-hexene
Which bond would you expect to break first in this molecule?
Sigma bond in C-H single bond
Pi bond in C-C double bond
Sigma bond in C-C double bond
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The Carbon-Carbon double bond is made of 1 sigma and 1 pi bond (because, double bond includes a pi bond). Sigma bonds are strongest. So, pi bonds will break first. Other bonds are only 1 sigma, whereas the Carbon-Carbon double bond is is 1 sigma + 1 pi. This makes it the strongest. In this, the pi bond will break first.