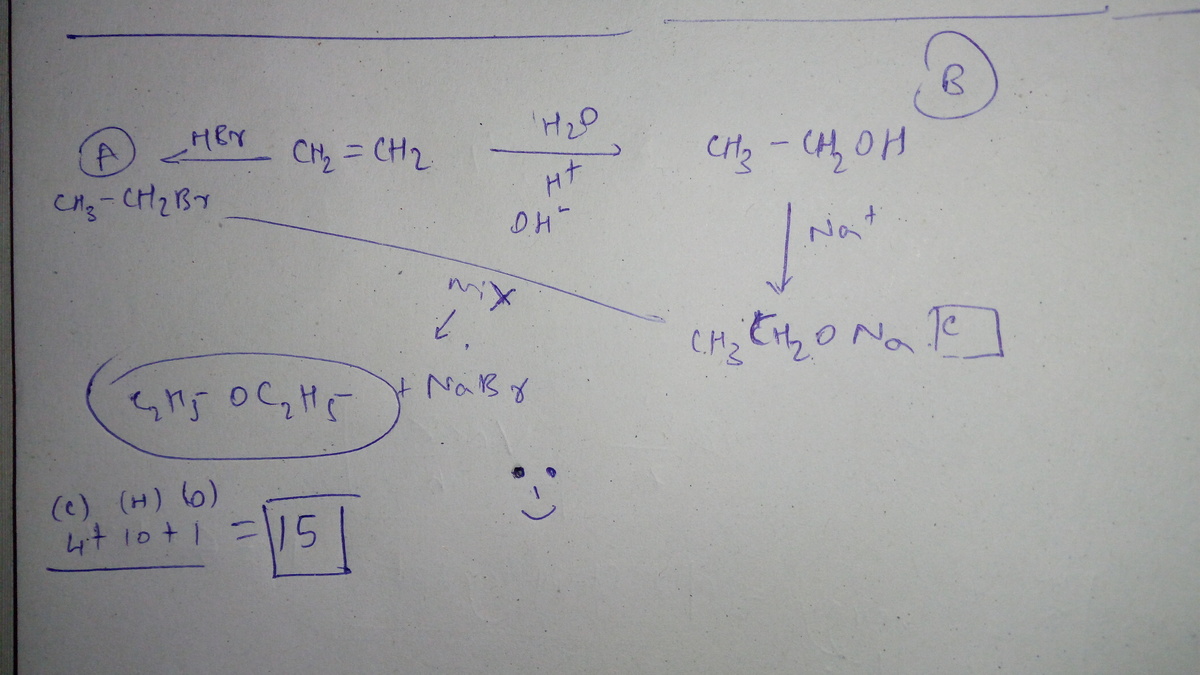
Simple Ethene Processing
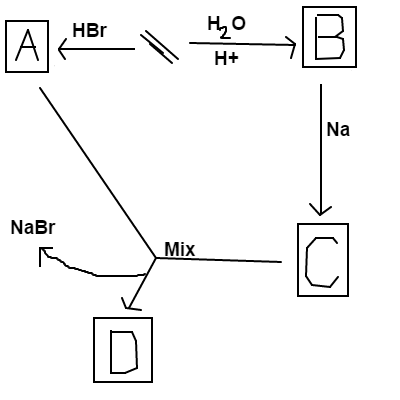 Follow the reaction scheme above, starting with ethene.
Give your answer as the total number of carbon, oxygen, bromine, and hydrogen atoms in
D
, the finished product of the reaction scheme above.
Follow the reaction scheme above, starting with ethene.
Give your answer as the total number of carbon, oxygen, bromine, and hydrogen atoms in
D
, the finished product of the reaction scheme above.
David's Organic Chemistry Set
David's Physical Chemistry Set
The answer is 15.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
2 solutions
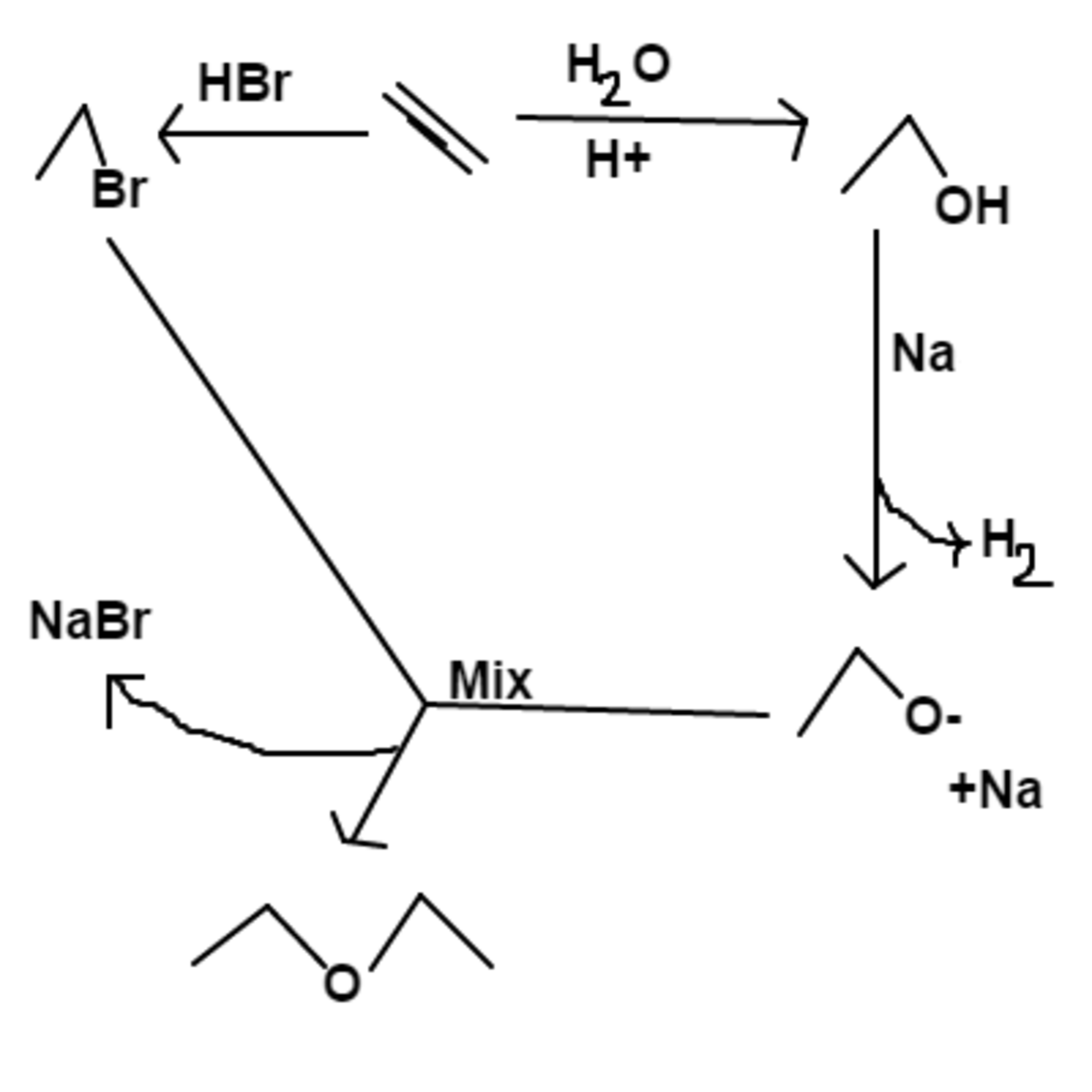 The formula of
D
, a.k.a.
diethyl ether
, is
(
C
2
H
5
)
2
O
; thus the total number of atoms in
D
is
1
5
.
The formula of
D
, a.k.a.
diethyl ether
, is
(
C
2
H
5
)
2
O
; thus the total number of atoms in
D
is
1
5
.
Ethanol will NOT react with naoh as ethoxide is stronger base than naoh which will not remain on product side. The substitution you want to carry out would occur directly producing HBr as a by product
Log in to reply
Yeah the substitution can directly occur . but in order to enhance the rat eof reaction we should add some strong base (capable enough to react with ethanol)
N a O H has been replaced with N a metal, which is capable of removing the hydrogens on alcohols. Thank you for bringing up the issue.
Yes right!. you should use stronger base like sodamide,Lithium di isopropyl amide etc
Log in to reply
Thanks for bringing that up. I replaced N a O H with N a metal which is capable of stripping hydrogens from alcohols.
bhai na dalne se oh - kyo nahi nikla h+ kyo nikla ? ( i have read it that alcohols give sodium alkoxide on reaction with Na plz tell me why?)