So Radioactive!
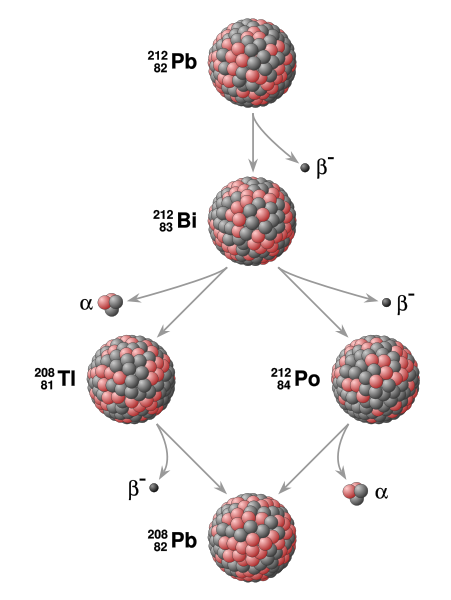
The half life of Pb(212) is 10.6 hours. It undergoes decay to its daughter nucleus Bi(212) which has a half life of 60.5 minutes. Calculate the time in minutes (to the nearest minute) at which the rate of the reaction is maximum, i.e., rate of disintegration of Pb(212) to the final product is maximum.
Image credit: Wikipedia
The answer is 227.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
N A and N B are the numbers of Pb and Bi atoms respectively.
 Nice problem buddy. Loved solving it. :)
Nice problem buddy. Loved solving it. :)