Spring with Thermodynamics
Two heat-insulating pistons connected by spring can slide without friction inside a horizontal heat insulating tube, which is open at both of its ends. One mole of a mono-atomic gas is filled between the pistons at temperature
At this temperature of the gas, the spring is not relaxed. If an amount of heat is supplied to the gas, its temperature becomes and the length of spring becomes times of its initial length.
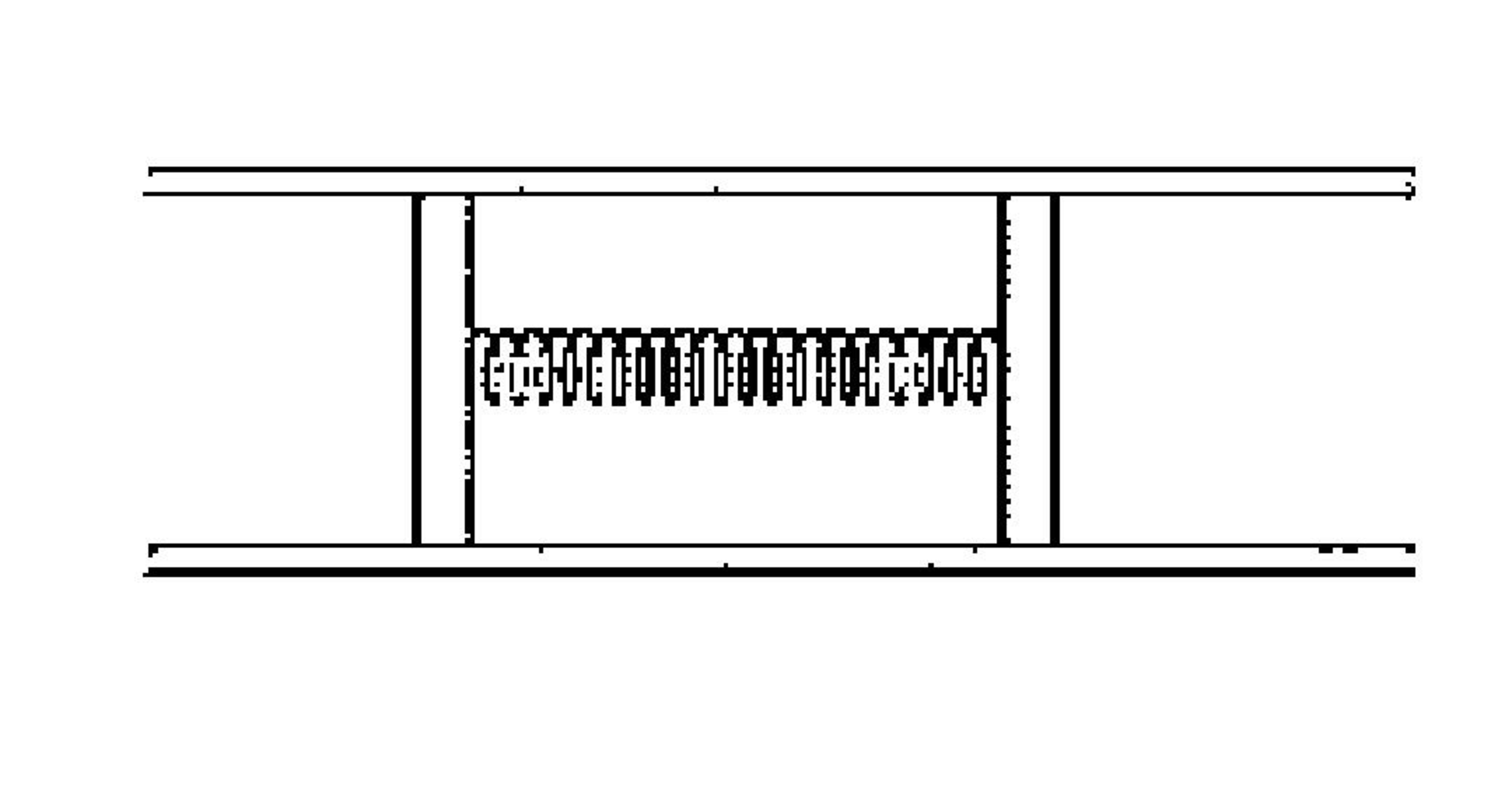
If the heat is supplied to the gas can be expressed as
submit your answer as the sum of constants
The answer is 2.5.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!