Structural Elucidation via NMR Spectroscopy & ESI-MS
The following 1H and 13C NMR spectra were acquired on a Bruker AVIII-400 NMR Spectrometer, equiped with a multinuclear 5 mm ATMA BBFO (13C/X-channel optimized) probe. The solvent used was chloroform-d.
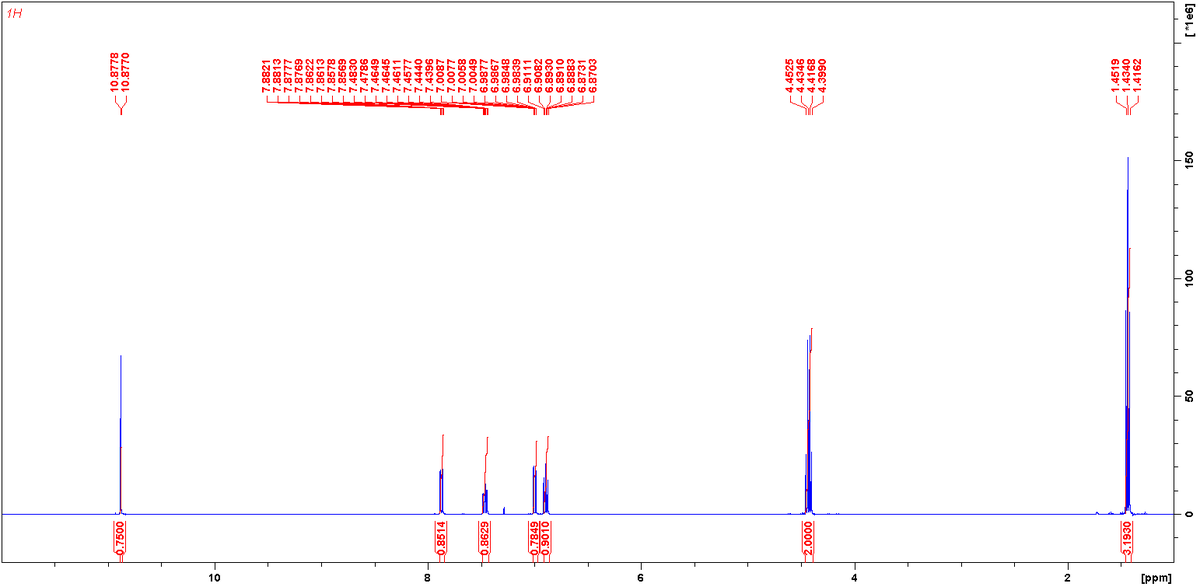
1H Spectrum (standard 90 deg pulse):
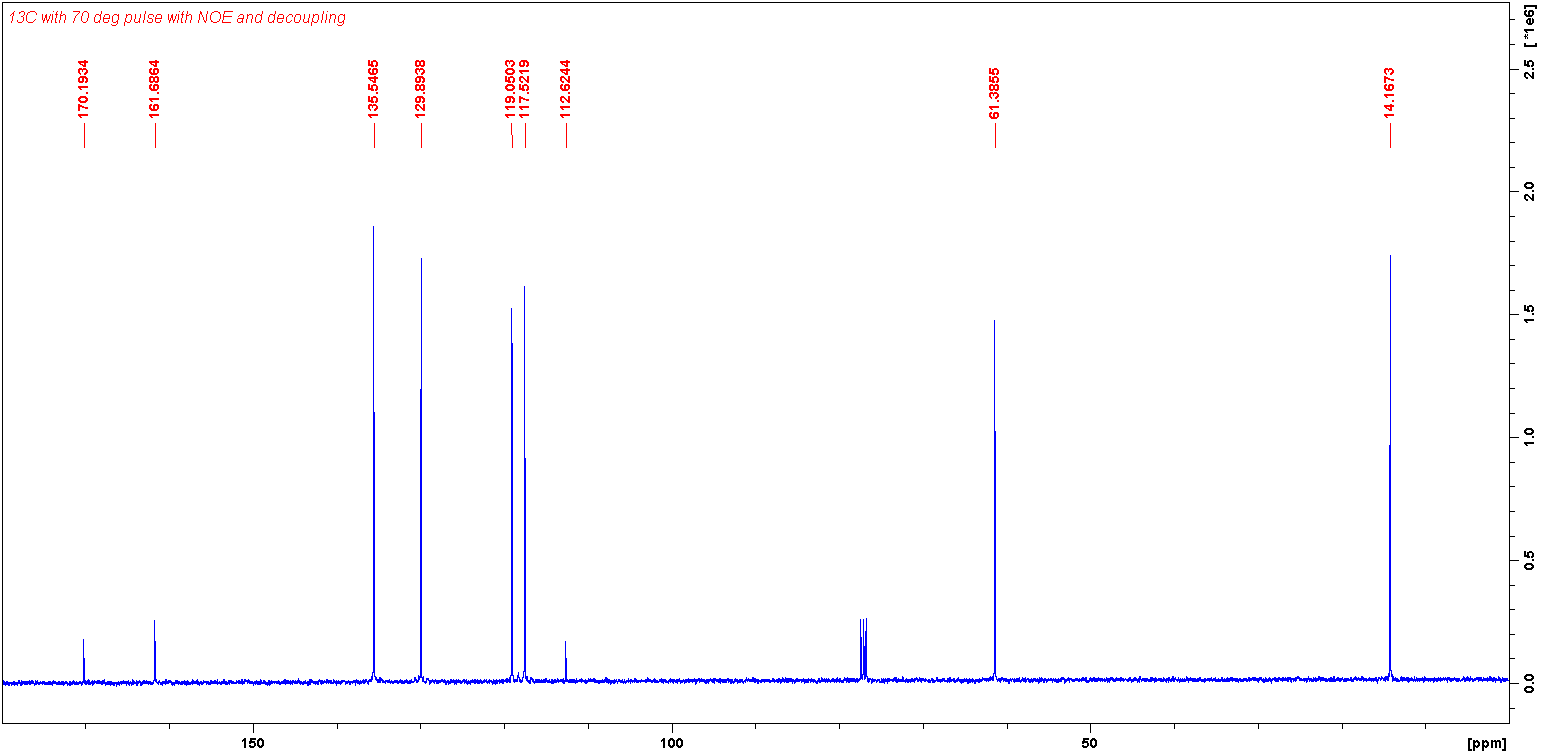
13C Spectrum (70 deg pulse with NOE and decoupling):
Given that a sodium adduct in a ESI mass spectrum of the molecule was observed at m/z = 189.15, which of the following molecules gives rise to the above NMR spectra?
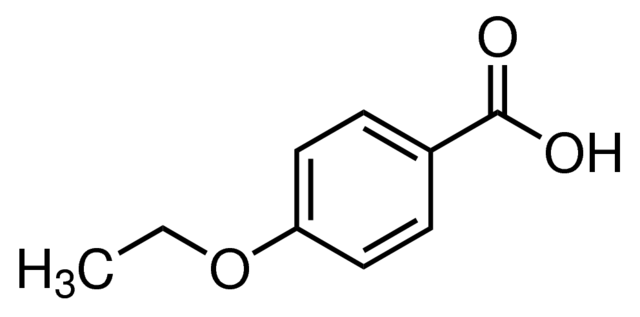
A)
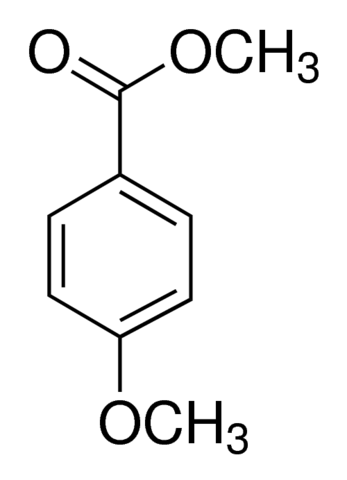
B)
C)
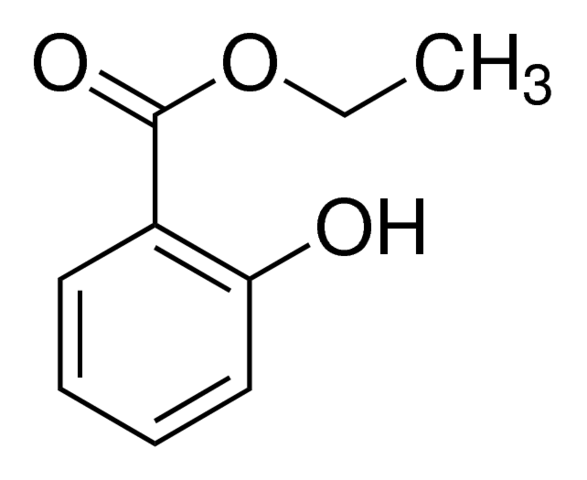
D)
Extra Help: Below is the FT-IR spectrum of the molecule to aid in your answering of the question.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Firstly, molecule (D) cannot be the correct molecule, as there are nine carbon resonances observed in the 13C NMR spectrum, and molecule (D) only has 7 carbons.
Molecule (B) cannot be the correct molecule, as it has two methoxy moieties, which would give rise to two closely spaced singlets in the 1H spectrum. This is not observed, thus molecule (B) cannot be the correct molecule.
Molecule (A) cannot be the correct molecule, as it has only 2 distinct proton environments in the aromatic ring (which would appear as two 1:1 doublets), yet the provided 1H spectrum clearly exhibits 4 distinct resonances in the aromatic region.
The molecule that gives rise to the provided NMR spectra is molecule (C): ethyl 2-hydroxybenzoate (ethyl salicylate). The molecular structure of ethyl salicylate is shown below.
The terminal methyl group is adjacent to a methylene group, therefore (n + 1) = 3. A 1:2:1 triplet is observed at ca. 1.4 ppm, with an relative integral of 3. Thus, this is assigned as the methyl peak.
The methylene group is adjacent to a terminal methyl, and an ester oxygen. Therefore (n + 1) = 4. A 1:2:2:1 quartet is oberved at ca. 4.4 ppm, with a relative integral of 2. Thus, this is assigned as the methylene peak.
The aromatic ring possesses 4 distinct proton environments, two of which will appear as doublets, as they are only adjacent to 1 proton bearing carbon atom. Four proton resonances are observed in the 1H spectrum in the aromatic region, two of which are doublets. (Note: the aromatic proton resonances are slightly split further via long-range coupling).
The hydroxyl proton will naturally appear as singlet, which is observed in the 1H spectrum at ca. 10.87 ppm.
To assign the spectrum of ethyl salicylate from first principles, one would need to acquire a set of different NMR experiments, including: 1H,1H-COSY, DEPT(45, 90 and 135), HMBC, HSQC, and possibly SELTOCSY spectra. But as the other three molecules could be excluded by using simple chemical logic, these were not required to answer the question.