Tert-Butane Reaction Scheme
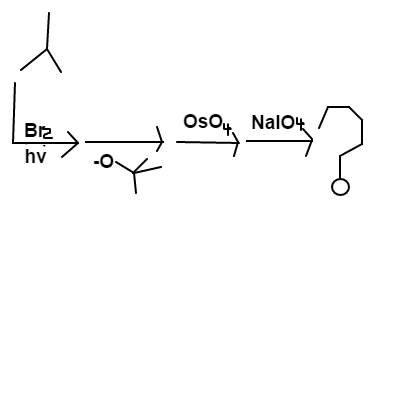 In the reaction scheme of
shown above,
is first reacted with
in light, followed by a treatment with
. The major product is then mixed with
; assume this is followed by addition of a reducing agent (
). Lastly,
is mixed in.
In the reaction scheme of
shown above,
is first reacted with
in light, followed by a treatment with
. The major product is then mixed with
; assume this is followed by addition of a reducing agent (
). Lastly,
is mixed in.
Give your answer as the total number of , , and in the final major product(s) of this reaction scheme.
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
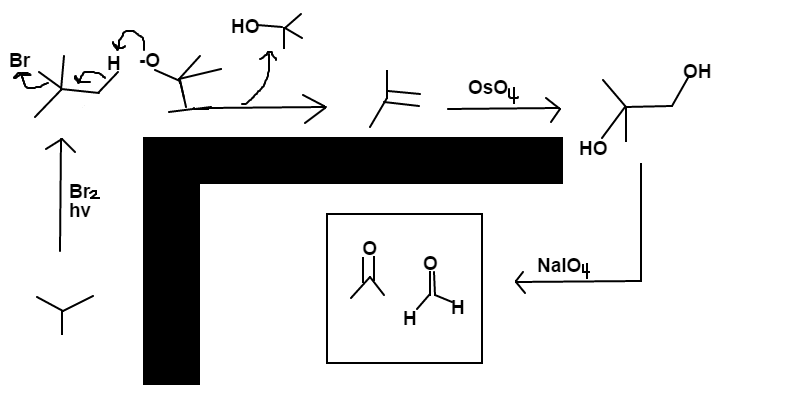 The final major products are isopropanone (
C
3
H
6
O
) and methanal (
C
H
2
O
).
Answer:
=
1
4
The final major products are isopropanone (
C
3
H
6
O
) and methanal (
C
H
2
O
).
Answer:
=
1
4