Tessellate S.T.E.M.S - Physics - College - Set 3 - Problem 2
In the cycle AEBFA given below, both AEB and BFA are reversible processes. Also, during the process AEB, the system (only)absorbs heat and during the process BFA , the system (only) releases heat , i.e. and . Then which of the following statements are correct ?
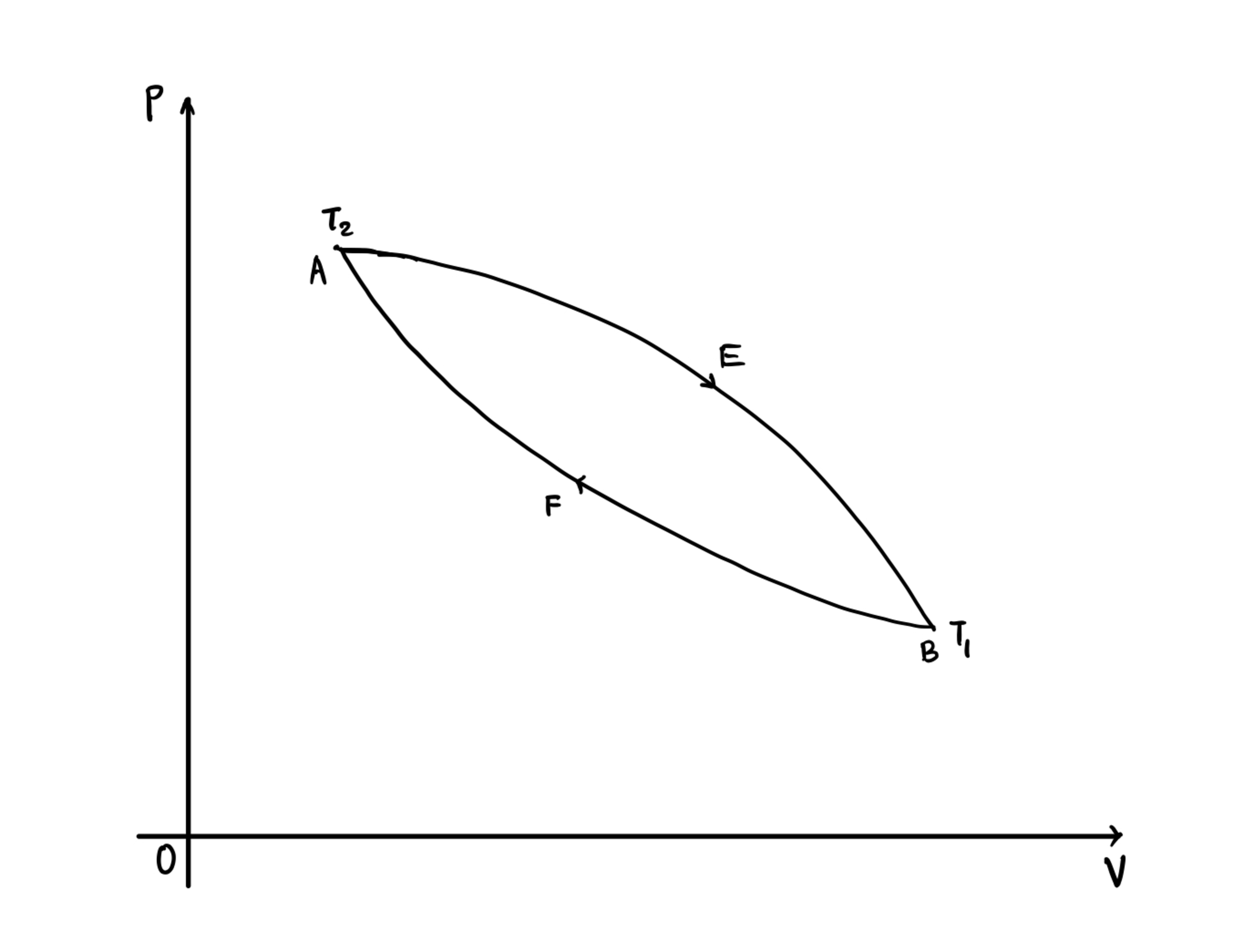
Entropy of the system increases.
The total work done equals to the net heat absorbed .
Efficiency of the cycle is independent of the type of reversible process.
Efficiency of the cycle is unchanged irrespective of the reversibility condition
This problem is a part of Tessellate S.T.E.M.S.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
(i) Entropy is a state variable, so is independent on the path taken to reach the state
(ii) Entropy can't increase if you start and end in the same place! Is a state variable!
(iii) Internal energy is a state variable, so it has to be the same if you are in the same point. Change in internal energy equals heat absorbed - work done by system (Or heat absorbed and work done one the system if you want). Since the change is zero, both work done by the system and heat absorbed have to be equal
Read more