The Kingdom of Chemithia- Part 4
The crowd in the town square is massive as they wait for King Antimony to come outside to announce the new Royal Chemists. Somewhere, squashed between the hundreds of people is you. Seeing all the people, you become a little tense about your chances. Suddenly everyone cheers as King Antimony steps out onto the palace balcony, dressed in a fine velvet robe. "Citizens of Chemithia! In the name of our Prophets St. Bohr and St. Mendeleev, we are gathered here today to select the next set of 7 Royal Chemists!"
Everyone cheers. King Antimony takes out a scroll and unrolls it. You seem to hear the entire crowd gulp at the same time. "The first Royal Chemist is..." he proclaims, "Ferric Selenian!" A huge cheer from a faraway part of the crowd erupts, and a small, wiry man with wide-rimmed glasses jostles his way through the crowd to stand next to King Antimony. As King Antimony gives the mantle to Vanadis Ionica, Niobe Hass, Wolf Clemmenson, Corey House and Kharasch Saytzeff, you begin to worry. Only one more spot left!
Finally, King Antimony reads out the last chemist. "Aurous Promethean!" A cheer goes up. You feel faint. It takes you what feels like an eternity to collect yourself and push through the crowd to the revered altar. You can't believe it. You're a Royal Chemist!
King Antimony gives a speech about development and hope for the future then bids you to come with him to the palace. The seven of you follow in an orderly line. King Antimony enters the palace and tells you to wait outside. Then a guard shows up. Dressed in green and yellow, he says that he has been commanded to give 'a final test to the new chemists'.
He begins asking each of you one question, and the first six shuffle into the palace, leaving only you. He asks you the following question: What is the main reason for the melting point of Stannic tetrachloride ( ) being less than stannous chloride ( )?
Note: Stannic ion is
This question is part of my set Tales of Chemithia ! Give it a try; it's a story woven together by Chemistry problems!
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
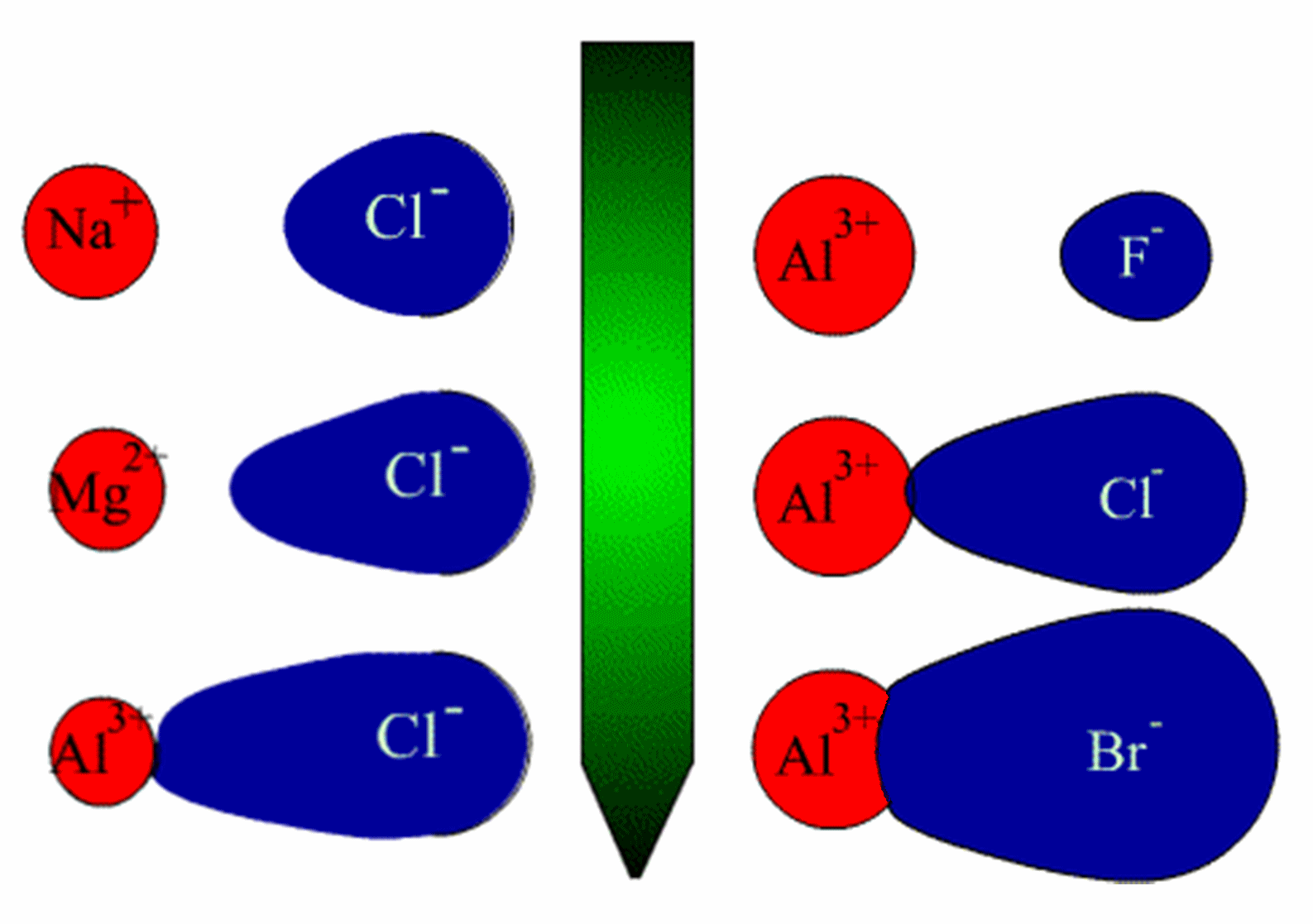 Tin(II) chloride has a higher melting point because tin(II) has a fairly low charge density. The bonding in
S
n
C
l
2
will be predominantly ionic. The
high charge density of the tin(IV) ion would result in covalent bonding for
S
n
C
l
4
and therefore a low melting point. This can be elaborated on the point of polarizing power of the cation and polarisability of the anion that gives rise to the unique covalent character in the
S
n
C
l
4
compound which we would expect to be an ionic compound that would supposedly have higher melting point but clearly, consideration of just the higher cationic charge on the lattice energy in
S
n
C
l
4
will not do here in this comparison between
S
n
C
l
4
and
S
n
C
l
2
. You can take a look at how the polarisability works in the above picture comparison.
Tin(II) chloride has a higher melting point because tin(II) has a fairly low charge density. The bonding in
S
n
C
l
2
will be predominantly ionic. The
high charge density of the tin(IV) ion would result in covalent bonding for
S
n
C
l
4
and therefore a low melting point. This can be elaborated on the point of polarizing power of the cation and polarisability of the anion that gives rise to the unique covalent character in the
S
n
C
l
4
compound which we would expect to be an ionic compound that would supposedly have higher melting point but clearly, consideration of just the higher cationic charge on the lattice energy in
S
n
C
l
4
will not do here in this comparison between
S
n
C
l
4
and
S
n
C
l
2
. You can take a look at how the polarisability works in the above picture comparison.